Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the 2019–2020 coronavirus disease (COVID-19) pandemic, invades host cells via the angiotensin-converting enzyme 2 (ACE2) receptor, resulting in direct cellular injury. ACE2 is highly expressed in the respiratory tract, particularly in type II alveolar epithelial cells, conferring strong tropism to the lungs. However, it is also distributed throughout the body—including vascular endothelium, the heart, kidneys, liver, and gastrointestinal tract—rendering multiple organs susceptible to infection [1–4].
SARS-CoV-2 infection disrupts the regulatory role of ACE2 within the renin–angiotensin–aldosterone system (RAAS), promoting vasoconstriction and fibrosis. Endothelial infection creates a prothrombotic milieu that accelerates coagulation cascade activation and microthrombus formation. In addition, dysregulation and hyperactivation of the innate immune system trigger cytokine release syndrome and hyperinflammatory responses [3,4].
In children and adolescents, COVID-19 is usually asymptomatic or presents with mild symptoms such as fever, cough, or dyspnea [5]. However, several weeks after SARS-CoV-2 infection, some pediatric patients have developed a hyperinflammatory condition involving multiple organs. The World Health Organization (WHO) has defined this novel syndrome as multisystem inflammatory syndrome in children (MIS-C) [6].
MIS-C generally appears 2–6 weeks after SARS-CoV-2 infection and is thought to arise from a dysregulated immune response rather than direct viral injury [7]. MIS-C can involve cardiovascular compromise, with many patients presenting with hypotension and myocardial dysfunction. This requires early evaluation of cardiac involvement, timely initiation of immunomodulatory treatment, and continued cardiac follow-up [8]. In this study, we present two pediatric cases of MIS-C to illustrate their clinical characteristics and diagnostic evaluation, describe the therapeutic strategies used, and offer insight into the potential pathophysiological mechanisms.
Case Report
A 9-year-old girl presented with a 7-day history of fever and upper respiratory symptoms. She had been diagnosed with otitis media and lymphadenitis at a local clinic and was treated with clarithromycin, amoxicillin/clavulanate, and a third-generation cephalosporin. She developed persistent fever reaching 39°C, abdominal pain, vomiting, and urinary symptoms, accompanied by conjunctival injection, swelling of the hands and feet, and a truncal rash suggestive of Kawasaki disease (KD).
Laboratory tests showed a prolonged prothrombin time (INR 1.34) and elevated inflammatory markers, including C-reactive protein (CRP, 46.4 mg/L), procalcitonin (0.52 ng/mL), and serum amyloid A (SAA > 300 mg/L). Cardiac biomarkers were markedly elevated, with an NT-proBNP level of 1,616 pg/mL. A respiratory pathogen panel detected rhinovirus A/B, metapneumovirus, Mycoplasma antibodies, and SARS-CoV-2 antibodies. Echocardiography revealed reduced left ventricular (LV) systolic function (ejection fraction 43%) while right ventricular (RV) function remained preserved. No coronary artery dilation, thrombus, or infarction was identified on echocardiography (Fig. 1).
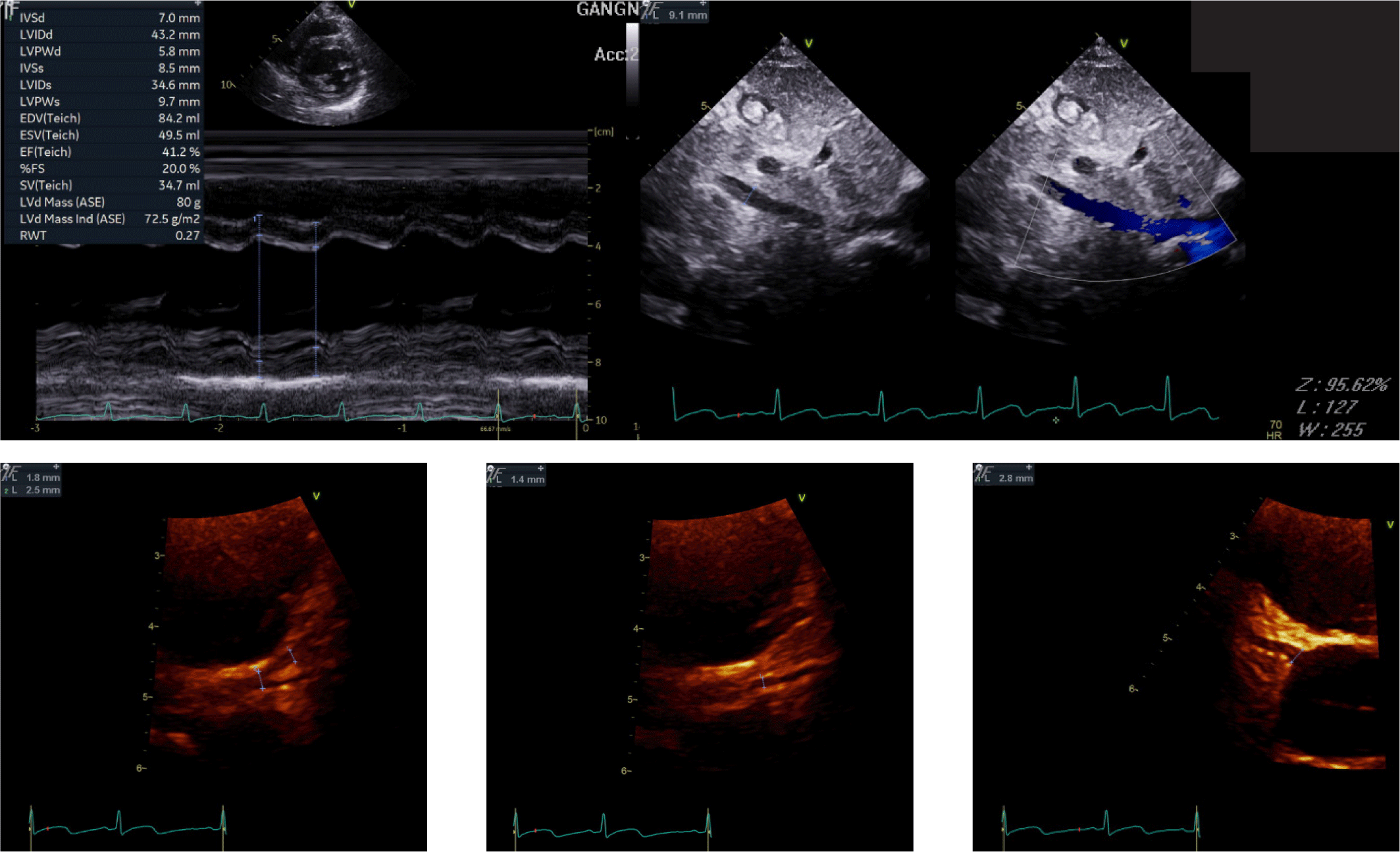
According to diagnostic criteria, KD requires fever lasting ≥ 5 days and at least four of the five principal features (conjunctival injection, oral mucosal changes, cervical lymphadenopathy, truncal rash, and extremity changes). When only two to three criteria are met, incomplete KD can be diagnosed if there are supportive findings such as coronary artery involvement, elevated inflammatory markers, anemia, or thrombocytosis. Although the patient had fever for more than five days and met three principal criteria, the absence of coronary complications made KD unlikely.
Given that the patient (aged < 19 years) had persistent fever for more than three days, rash, conjunctivitis, swelling of the hands and feet, reduced LV ejection fraction, prolonged PT, gastrointestinal symptoms, elevated inflammatory markers, and serologic evidence of SARS-CoV-2 infection, the diagnosis of multisystem inflammatory syndrome in children (MIS-C) was established.
She was treated with intravenous immunoglobulin (IVIG) and methylprednisolone as first-line therapy, along with aspirin for thromboprophylaxis [5,9,10]. After clinical improvement, steroids were tapered and switched to oral formulation before discharge. Follow-up showed normalization of cardiac function [left ventricular ejection fraction (LVEF) = 65%], preserved RV function, and no coronary artery complications.
An 8-year-old girl with no significant perinatal or medical history presented with fever and respiratory symptoms. She had undergone dental extraction one month before symptom onset and had close contact with relatives about one week prior. She developed a fever and upper respiratory symptoms and took over-the-counter medications and antipyretics. Two days later, she complained of chest tightness and heaviness, along with persistent general weakness. Laboratory testing at a local hospital revealed markedly elevated cardiac biomarkers—troponin T, troponin I, creatine kinase (CK), CK-MB, and NT-proBNP—raising suspicion for cardiomyopathy, infective myocarditis, or infective endocarditis, and she was referred to our institution for further evaluation.
On admission, laboratory tests showed significantly elevated cardiac enzymes: troponin I 1,230 pg/mL, troponin T 530 pg/mL, and NT-proBNP 16,933 pg/mL, while inflammatory markers were within the normal range. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were both elevated (112 and 134 IU/L, respectively), suggesting possible viral infection of the respiratory or gastrointestinal tract (e.g., Coxsackievirus or SARS-CoV-2). However, viral serologies (Mycoplasma IgM/IgG, EBV VCA IgM/IgG) and respiratory viral polymerase chain reaction (PCR) testing (including rhinovirus, enterovirus, and SARS-CoV-2) were all negative, and bacterial cultures showed no growth. Iron studies showed iron deficiency anemia with serum iron 16 μg/dL and iron saturation of 6%.
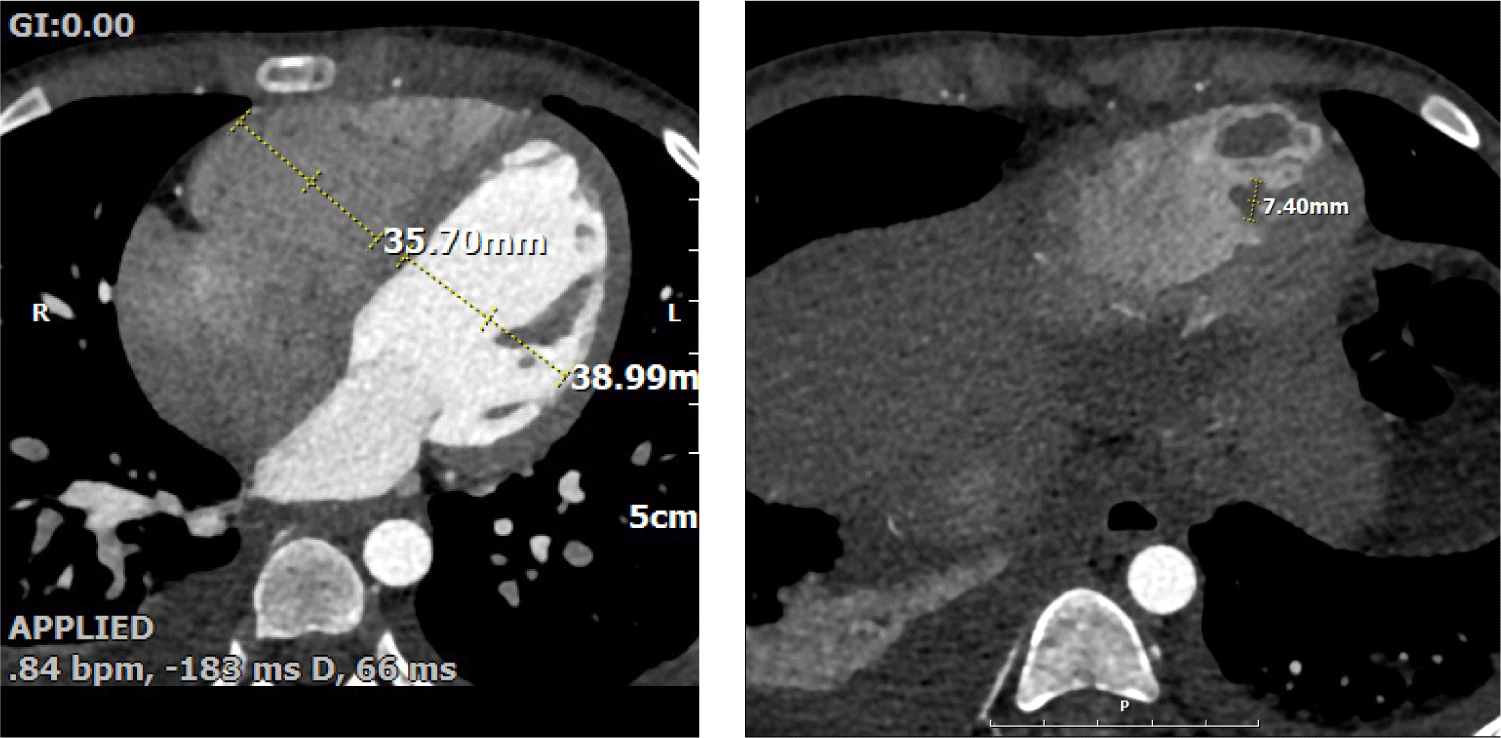
Echocardiography revealed impaired LV systolic function (LVEF 43%, global longitudinal strain −12.5%) and RV dysfunction (TAPSE 10 mm, tricuspid annular S′ 9 cm/s, RVFAC 20%). The inferior vena cava was mildly dilated (17 mm) with poor inspiratory collapse, also indicating deteriorated RV function. A large oval mass (1.5 × 0.7 cm) was visualized at the RV apex (Fig. 2). The mass was suspected to represent either vegetation or thrombus, and cardiac computed tomography (CT) findings favored thrombus over vegetation (Fig. 3). It was hypothesized that a small vegetation may have developed secondary to infection, while reduced RV systolic function caused turbulent flow; together with the prothrombotic tendency associated with SARS-CoV-2 infection, these factors likely accelerated the formation of a larger thrombus.
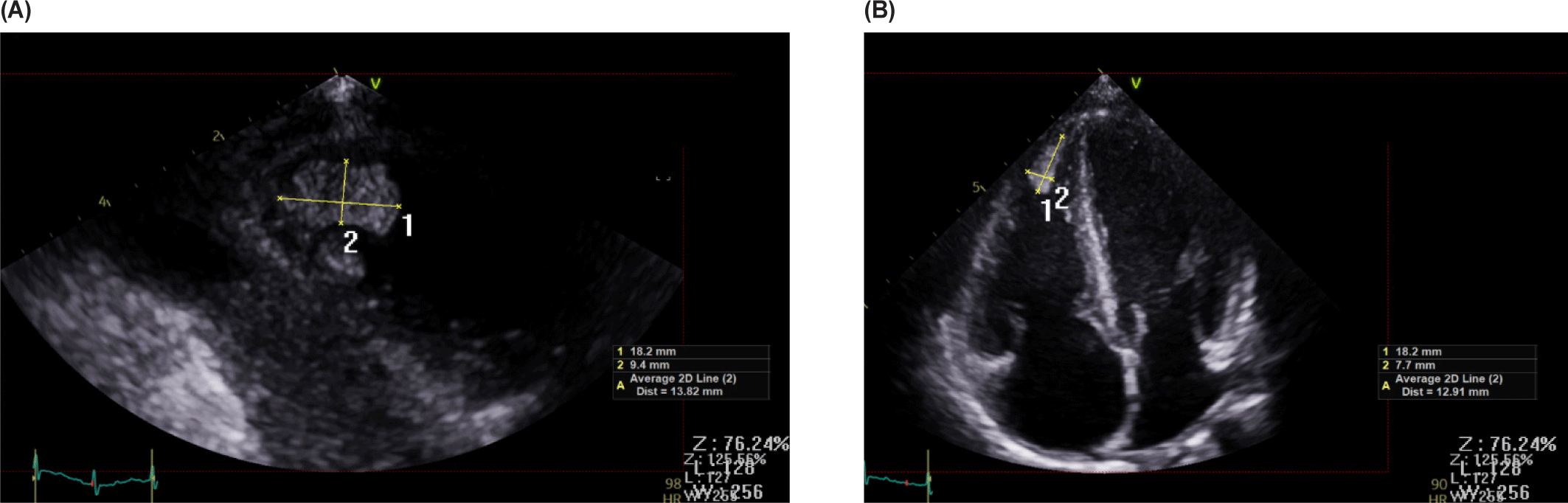
Blood cultures were negative; however, the combination of fever, vascular manifestations, and echocardiographic findings supported a clinical diagnosis of infective endocarditis. Cardiac involvement was also evident, as elevated cardiac biomarkers, chest pain, and impaired ventricular function suggested concurrent myocarditis. The patient’s iron-deficiency–associated immune vulnerability, recent dental procedure, and close household contact were considered possible sources of infection.
Empiric antimicrobial therapy with ampicillin/sulbactam and ceftriaxone was initiated, and oral doxycycline was subsequently added to cover a potential Mycoplasma-associated myocarditis or endocarditis. An antiviral agent (isoprinosine) was also administered. These agents were combined with immunomodulatory therapy using intravenous methylprednisolone and IVIG [9]. Because the RV mass was likely thrombotic, anticoagulant therapy with aspirin, heparin, and warfarin was initiated, along with iron supplementation for anemia. The patient’s cardiac biomarkers normalized, echocardiography showed regression of the RV mass and recovery of biventricular function, and she was discharged after tapering steroids and discontinuing antimicrobial therapy.
One month later, she returned with chest discomfort and generalized weakness. Laboratory tests showed increased NT-proBNP (619 pg/mL) and troponin levels. A respiratory viral panel detected coronavirus, and both SARS-CoV-2 N and S antibodies were present. Given the fever lasting five days, reduced LV function, elevated cardiac biomarkers, and confirmed SARS-CoV-2 infection, a diagnosis of MIS-C was confirmed. She received a 10-day course of corticosteroids and recovered completely without any sequelae.
The diagnostic criteria for MIS-C were reviewed for both patients, and the outcomes are shown in Tables 1 and 2.
| MIS-C definition [6] | Case 1 | Case 2 |
|---|---|---|
| Children 0–19 years of age Fever > 3 days |
F/ 9 years Fever for 7 days |
F/8 years Fever for 5 days |
| AND two of the following: 1. Rash or conjunctivitis or mucocutaneous inflammation signs 2. Shock or hypotension 3. Features of myocardial dysfunction, valvulitis, pericarditis, and coronary abnormalities (elevated Troponin/NT-proBNP or ECHO findings) 4. Evidence of coagulopathy (by PT, PTT, d-Dimers) 5. Acute gastrointestinal problems (diarrhea, vomiting, abdominal pain) |
1. Truncal rash, conjunctivitis, hand/feet swelling 3. NT-proBNP ↑, LV function↓ (EF 43%) 4. PT prolongation 5. Abdominal pain, vomiting |
3. LV function↓ (EF 43%) troponin/NT-proBNP ↑ |
| AND elevated markers of inflammation (ESR, CRP, procalcitonin) | CRP 46.6 mg/L procalcitonin 0.52 ng/mL | (–) |
| AND No other obvious microbial cause of inflammation |
Mycoplasma Ab (+) Rhinovirus (+) Metapneumovirus (+) |
(–) |
| AND evidence of COVID-19 (antigen test, serology or RT-PCR +) or contact with patients with COVID-19. | SARS-CoV-2 Ab (+) | RT-PCR (+) SARS-CoV-2 Ab (+) |
| Shares common features with other pediatric inflammatory conditions including: KD, TSS, bacterial sepsis, MAS | Kawasaki disease | Kawasaki disease |
| Diagnosis | MIS-C | MIS-C, Infective endocarditis Infective myocarditis |
| Management | 1. IVIG 60 g IVF (5/14) 2. Aspirin 80 mg QD (5/15-) 3. IV methylprednisolone 20 mg q12 hr (5/16–5/19) |
1. Antiviral, Antibiotics 2. Methyprednosolone 3. IVIG 4. Aspirin, Heparin, warfarin 5. ACE inhibitor, beta-blocker 6. Iron replacement |
MIS-C: multisystem inflammatory syndrome in children, WHO: World Health Organization; IVIG: intravenous immunoglobulin, IVF: intravenous fluid, KD: Kawasaki disease, TSS: toxic shock syndrome, MAS: macrophage activation syndrome, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, PT: prothrombin time, aPTT: activated partial thromboplastin time, EF: ejection fraction, ECHO: echocardiography, NT-proBNP: N-terminal pro-B-type natriuretic peptide, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, COVID-19: coronavirus disease 2019, RT-PCR: reverse transcription polymerase chain reaction, Ab: antibody, (–): absent or not available, (+): positive; QD: quaque die; ACE; angiotensin-converting enzyme.
| MIS-C definition [11] | Case 1 | Case 2 |
|---|---|---|
| Clinical criteria All of the following criteria must be met 1. Person aged < 21 2. Fever (temperature ≥ 38.0 °C) 3. Clinical severity requiring hospitalization or resulting in death 4. Evidence of systemic inflammation (CRP ≥ 3 mg/dL) 5. New onset manifestations in at least two of the following categories: 1) Cardiac involvement - Left ventricular ejection fraction < 55% OR - Coronary artery dilatation, aneurysm, ectasia OR - Troponin elevated above laboratory normal range 2) Mucocutaneous involvement - Rash OR - Inflammation of the oral mucosa OR - Conjunctivitis or conjunctival injection OR - Extremity findings (e.g., erythema or edema) 3) Shock 4) Gastrointestinal involvement - Abdominal pain OR Vomiting OR diarrhea 5) Hematologic involvement - Platelet count < 150,000 cells/μL OR - Absolute lymphocyte count (ALC) < 1,000 cells/μL |
1. F/9 years 2. Fever for 7 days 3. Yes 4. CRP 46.6 mg/L 5. Cardiac involvement (LVEF 43%), mucocutaneous involvement (truncal rash, conjunctivitis, hand, feet swelling), gastrointestinal involvement (abdominal pain, vomiting) |
1. F/8 years 2. Fever for 5 days 3. Yes 4. CRP 1.7 mg/L 5. Cardiac involvement (LVEF 43%, troponin elevation) |
| No other obvious microbial cause of inflammation | Mycoplasma Ab (+) Rhinovirus (+) Metapneumovirus (+) |
|
| Laboratory criteria Evidence of SARS-CoV-2 infection, demonstrated by detection of viral RNA, antigen, or specific antibodies in clinical or post-mortem specimens within 60 days prior to or during hospitalization1) |
SARS-CoV-2 Ab (+) | RT-PCR (+) SARS-CoV-2 Ab (+) |
| Epidemiologic linkage Close contact2) with a confirmed or probable case of COVID-19 disease in the 60 days prior to hospitalization |
1) Includes a positive serology test regardless of COVID-19 vaccination status. Detection of anti-nucleocapsid antibody is indicative of SARS-CoV-2 infection, while anti-spike protein antibody may be induced either by COVID-19 vaccination or by SARS-CoV-2 infection.
Discussion
Case 1 involved a 9-year-old girl with a seven-day history of persistent fever, accompanied by a rash, conjunctival injection, and swelling of the hands and feet. Laboratory evaluation demonstrated myocardial dysfunction, coagulation abnormalities, elevated inflammatory markers, and serologic evidence of SARS-CoV-2 exposure. Although rhinovirus A/B and human metapneumovirus were detected on the respiratory pathogen panel, these viruses are common causes of mild upper respiratory infections in children and are often identified incidentally. The patient did not report ongoing respiratory symptoms at the time of diagnosis, and overall clinical features—including myocardial dysfunction, markedly elevated cardiac biomarkers, gastrointestinal symptoms, conjunctival injection, and peripheral edema—were more consistent with MIS-C rather than typical manifestations of these respiratory viruses. In addition, Mycoplasma co-infection was identified but was also insufficient to explain the systemic inflammatory state. Based on these findings, the clinical diagnosis of MIS-C was made, and immunomodulatory therapy with IVIG and methylprednisolone was initiated. The patient showed prompt improvement in cardiac function and systemic inflammation following treatment, further supporting MIS-C as the primary diagnosis.
In Case 2, an 8-year-old girl had fever for five days, reduced cardiac function, and elevated myocardial enzymes, with prothrombin time at the upper normal limit. She tested positive for SARS-CoV-2 antibodies, and no other infectious agent was identified. Although the diagnostic criteria for MIS-C require elevated inflammatory markers, all inflammatory indices in this case were within normal limits. Considering that the patient had received multiple antimicrobial agents at three different hospitals before admission, we considered the possibility that inflammatory markers might have been attenuated. Therefore, the clinical picture was deemed compatible with MIS-C. Immunomodulatory therapy with IVIG and methylprednisolone led to rapid clinical improvement.
The clinical manifestations in Case 1—including conjunctivitis, truncal rash, and extremity edema—overlapped with those of KD. However, only three of the five principal diagnostic criteria for KD were met, and echocardiography revealed no coronary artery abnormalities, thereby excluding KD. In Case 2, although fever and cardiac involvement were present, there were no mucocutaneous findings characteristic of KD, including conjunctival injection, rash, extremity changes, or cervical lymphadenopathy. Therefore, KD was considered unlikely.
Although MIS-C and KD share similar clinical features, they are distinct syndromes that require careful differentiation [11,12]. KD occurs predominantly in Asian populations, whereas MIS-C is more prevalent among children of Hispanic or African descent. Compared with KD, MIS-C more frequently involves gastrointestinal and neurologic symptoms and exhibits a higher incidence of LV dysfunction and shock. Moreover, KD typically affects children under five years of age, whereas the median age for MIS-C onset is approximately 8.3 years, indicating that KD predominates in younger children, while MIS-C occurs more frequently after age five [7,10].
Differences in the epidemiologic patterns of MIS-C and KD may be partly related to age-dependent variation in ACE2 expression, the receptor used by SARS-CoV-2 to enter host cells. ACE2 is expressed in the lungs, heart, gastrointestinal tract, and notably in vascular endothelial cells, which could help explain the vasculitic features observed in some SARS-CoV-2–associated conditions.
Because ACE2 expression changes across developmental stages, the distinct age distributions of MIS-C and KD might suggest a possible shared pathophysiological pathway influenced by maturation-related factors [13]. Although the exact pathogenesis of KD remains unclear, one hypothesis is that an infectious trigger, along with its mediating mechanisms, may contribute to dysregulated immune activation and subsequent disease development.
Both cases demonstrated notable cardiac involvement, an important clinical feature of MIS-C. Although myocardial dysfunction and thrombotic complications can progress rapidly in MIS-C, cardiac involvement often improves with immunomodulatory treatment using IVIG and corticosteroids. These findings suggest that MIS-C should be suspected in children with cardiac involvement after SARS-CoV-2 exposure, and that immunomodulatory treatment should be started early. Ongoing cardiologic follow-up is also warranted due to the potential for delayed cardiac sequelae.







