Research and Publication Ethics
Enacted on December 28, 2022
Revised on July 1, 2025
Regarding policies on research and publication ethics not addressed in these instructions, authors should refer to the Committee on Publication Ethics (COPE) guidelines on good publication (https://publicationethics.org/guidance), the Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals by the ICMJE (https://www.icmje.org/recommendations/) or Good Publication Practice Guidelines for Medical Journals (KAMJE, https://www.kamje.or.kr/guide/books).
A. Statement of human and animal rights and informed consent
Any investigations involving humans and animals should be approved by the institutional review board (IRB) or institutional animal care and use committee (IACUC), respectively, of the institution where the study took place. In addition, investigations with pathogens requiring a high degree of biosafety should obtain approval from the relevant committee (institutional biosafety committee). Informed consent should be obtained, unless waived by the IRB, from patients (or legal guardians) who participated in clinical investigations. Human participants should not be identifiable, such that patients' names, initials, hospital numbers, dates of birth, or other protected healthcare information should not be disclosed. If experiments involve animals, the research should be based on national or institutional guidelines for animal care and use. Original articles submitted to KD that address any investigation involving humans and animals should include a description of whether the study was conducted under an approval by the IRB (with or without patient informed consent) or IACUC, respectively. IRB approval number is required for the submission process, and if absent, the process cannot proceed. When appropriate, the editorial office of KD may request official documentation of IRB or IACUC approval. All human studies are expected to follow the ethical principles outlined in the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/). It is noteworthy that a Korean act of bioethics and biosafety was revised on November 11, 2014.
B. Authorship and author’s responsibility
Authors must meet all aspects of the following 4 criteria:
(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data;
(2) Drafting the work or revising it critically for important intellectual content;
(3) Final approval of the version to be published;
(4) Agreement to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity are appropriately investigated and resolved.
Anyone who does not meet the above criteria may be listed as a contributor in the Acknowledgments section.
Only one corresponding author is allowed. A footnote indicating that two first authors contributed equally is permissible, as long as the authors certify equal first author role. The editorial board does not allow adding or changing authorship (including first or corresponding authors) after the manuscript has been accepted. Any change in the byline (such as addition or deletion of authors, or changing the order of names) requires a signed letter from all authors indicating their agreement. The editorial board assumes no responsibility for such changes.
The corresponding author takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication. They typically ensure that all the journal’s administrative requirements, such as providing authorship details, ethics committee approval, clinical trial registration documentation, and conflicts of interest (COI) forms and statements, are properly completed, although these duties may be delegated to one or more coauthors. The corresponding author should be available throughout the submission and peer review to respond to editorial queries. They should be available to respond to critiques and cooperate with journal requests for data, additional information, or questions about the paper even after publication. Authors may appeal the editorial decisions via e-mail. Appeals are processed according to procedures set by COPE (https://publicationethics.org/resources/discussion-documents/authorship).
C. Originality and duplicate publication
Manuscripts under review or published by other journals will not be accepted for publication in KD, and articles published in this journal are not allowed to be reproduced in whole or in part in any form of publication without the permission of the editorial board. Figures and tables can be used freely if original source is properly cited in accordance with the Creative Commons Non-Commercial License. It is mandatory for all authors to resolve any copyright issues when citing a figure or table from a different journal that is not open access.
D. Secondary publication
Manuscripts may be republished if they meet the ICMJE conditions for secondary publication as follows: certain types of articles, such as guidelines issued by governmental agencies and professional organizations, may need to reach the widest possible audience. In such instances, editors sometimes intentionally publish material that is simultaneously published in other journals, with the consent of the authors and the editors of those journals. Secondary publication, in the same or another language, especially in other countries, is acceptable and may be beneficial if the following conditions are met. The authors must obtain approval from the editors of both journals (the editor concerned with secondary publication must have a photocopy, reprint, or manuscript of the original article). The primary publication must be given precedence, with a minimum interval of one week before the secondary publication, unless a different arrangement is agreed upon by both editors.
The secondary publication should target a different audience; an abbreviated version may be appropriate. The secondary version must accurately reflect the data and interpretations of the primary publication. The footnote on the title page of the secondary version informs readers, peers, and documenting agencies that the paper has been published in whole or in part and cite the original publication. A suitable footnote might read: “This article is based on a study first published in [Journal Title], with full citation.”
E. Conflicts of interest (COI)
A COI may exist when an author, or the author’s institution or employer, has financial or personal relationships or affiliations that could bias the author’s decisions regarding the manuscript. All authors must disclose any relevant financial interests, or financial conflicts, including those that existed during the conduct of the research and at the time of publication. This includes future financial interests such as patent applications that may result in potential personal gain. All disclosures of any potential COI, including specific financial interests and relationships and affiliations (other than those affiliations listed in the title page of the manuscript) relevant to the subject of their manuscript will be disclosed by the corresponding author on behalf of each coauthor, if any, as part of the submission process. Likewise, authors without COI will be requested to state so as part of the submission process. If authors are uncertain about what constitutes a relevant financial interest or relationship, they should contact the editorial board. Failure to disclose COI will result in rejection or withdrawal of the manuscript. For all accepted manuscripts, each author’s disclosures of COI, relevant financial interests and affiliations, and declarations of no such interests will be published. The policy requesting disclosure of COI applies for all manuscript submissions. If an author’s disclosure of potential COI is determined to be inaccurate or incomplete after publication, an erratum will be published to rectify the original published disclosure statement. Authors are also required to report detailed information regarding all financial and material support for the research and work, including but not limited to grant support, funding sources, and provision of equipment and supplies as part of the submission process. For all accepted manuscripts, each author’s source of funding will be published.
The authors should disclose all potential COI. If there is a disclosure, the editors, reviewers, and readers can interpret the manuscripts with this understanding.
If a co-author is in a personal relationship with another (e.g., a person younger than 19 years, a spouse or a first-degree cousin), this should be marked in the byline with related facts written in a footnote. If necessary, the editorial board may request consent to provide personal information from the corresponding author, or an investigation from the affiliated institution. If a research misconduct by co-authors in a personal relationship is confirmed, the related fact can be notified to the relevant institution (e.g., advancement, employment, promotion, and research funding order, etc.) from which the author took advantage.
F. Process to manage research and publication misconduct
When the editorial board encounters suspected cases of research and publication misconduct-such as duplicate publication, plagiarism, fraudulent or fabricated data, changes in authorship, undisclosed COI, ethical concerns, appropriation of an author’s idea or data by reviewer, or complaints against editors, -the resolution process will follow the flowchart provided by COPE (https://publicationethics.org/guidance/Flowcharts). The Research Ethics and COI subcommittee is responsible for reviewing and making decisions on suspected cases. The Editor-in-Chief serves as the Chair of the Subcommittee, and its composition is publicly available on the journal’s website. In this process, a debatable matter may be consulted to KAMJE.
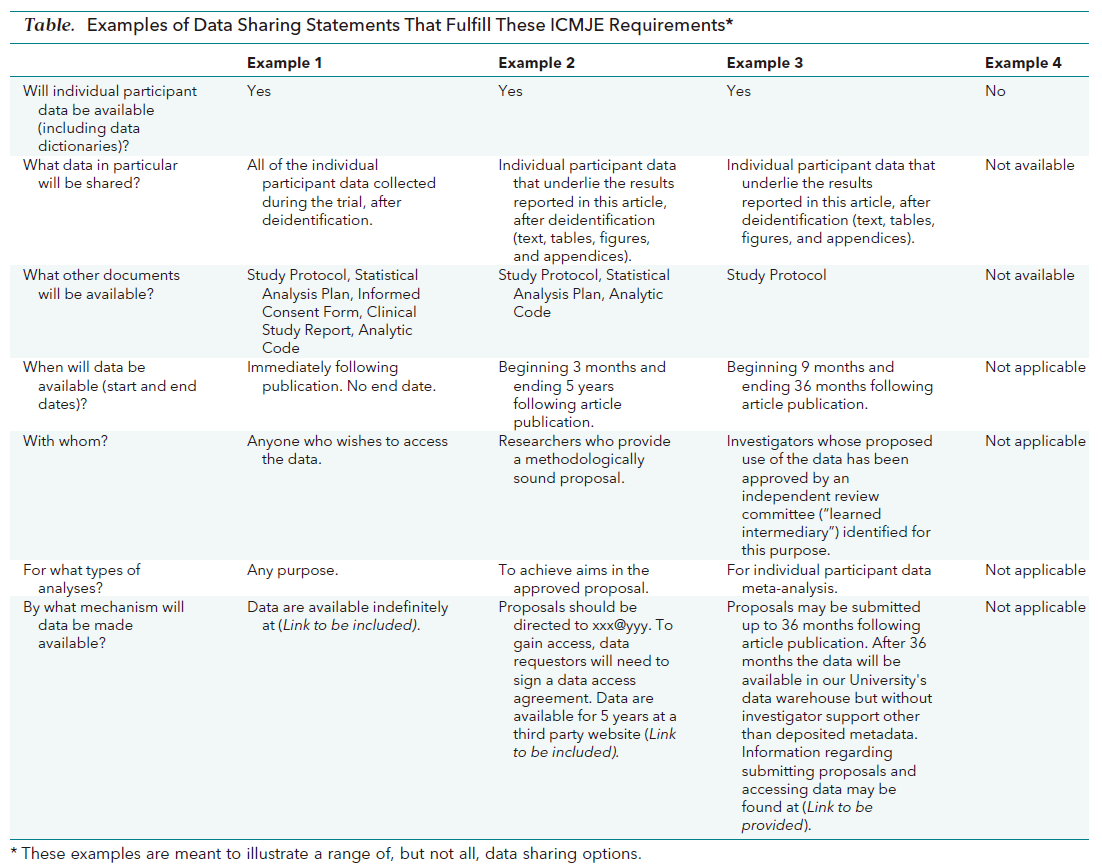
G. Data sharing
The journal encourages authors to state the data sharing in their submission. Authors may state linking to a repository or declaring confidentiality of the data. Since 2019, all manuscripts reporting clinical trials must be submitted with a data sharing statement (Table 1). If authors describe this in their manuscripts, the description will be published alongside their manuscripts.
Table 1. Examples of data sharing statements that fulfill the ICMJE requirements
H. Intellectual property
All published articles become permanent intellectual property of the KSKD and may not be republished elsewhere without written permission. Copyrights of the articles are owned by the KSKD. KD is an open-access journal. All articles are distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
I. Post-publication discussions and corrections
The research and publication ethics policy of KD is designed to protect the rights and integrity of authors, reviewers, editors, and the publisher. The post-publication discussion is available through correspondences. Readers can express concerns about a published article by submitting correspondence within 8 weeks of publication. If an error is identified in the article, it may be corrected through the author's reply, an erratum, or a retraction.
J. Preprint policy
KD allows the submission of manuscripts that have previously been posted on preprint servers. In such cases, authors should indicate the preprint server and DOI in the cover letter. Once the article is published in KD, the preprint should be linked to the final published KD version via its DOI (e.g., “This article has been published in Kawasaki Disease following peer review and can be viewed at [DOI].”), and the KD version should be cited, instead of the preprint. Citation of preprints is not permitted.
K. Use of artificial Intelligence (AI)
Authors may use generative AI and AI-assisted technologies (e.g., ChatGPT) to improve the clarity and language quality of their manuscripts. However, such tools must be employed with appropriate human oversight to ensure the accuracy and reliability of the output. Authors are responsible for carefully reviewing and editing any content produced by AI, as it may appear plausible but still contain errors. AI tools and technologies must not be credited as authors or cited as such, as authorship entails accountability and intellectual contributions that can only be assumed by humans. If generative AI was used during manuscript preparation, the name of the specific tool and the extent of its use must be clearly disclosed in the Methods or Acknowledgments section. This policy does not apply to the routine use of basic tools for grammar, spelling, reference checks, and similar editorial functions. Please note that authors are ultimately accountable for all aspects of the submitted work, including any use of AI or AI-assisted technologies. Adherence to this policy ensures that AI is used in a transparent, responsible, and ethically sound manner, consistent with best practices in scientific publishing.
L. Editorial responsibilities
The editorial board is committed to the ongoing monitoring and protection of publication ethics. This includes: establishing clear guidelines for article retraction; plagiarism screening for all manuscripts (https://www.ithenticate.com/); maintaining the integrity of the academic record; preclusion of business needs from compromising intellectual and ethical standards; publishing errata, clarifications, retractions and apologies when necessary; preventing plagiarism and fraudulent data. The responsibilities of the editorial board include: having the authority to accept or reject articles; avoiding conflicts of interest regarding decision; accepting a manuscript when reasonably certain of its validity; promoting the publication of erratum or retraction when errors are identified; and preserving the anonymity of reviewers.








