Introduction
The two types of natriuretic peptides—B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP)—have been utilized during the acute phase of Kawasaki disease for both diagnosis [1–3] and the prediction of outcomes following treatment [4–8]. The variation in natriuretic peptide levels is presumed to be primarily associated with myocarditis, which accompanies the majority of patients in the acute phase of Kawasaki disease, and several studies have reported numerical correlations with various parameters of cardiac function [9–11]. However, as myocardial involvement is often mild and subclinical in many patients, the elevation of natriuretic peptide levels has garnered attention for its clinical utility as a potential predictor of resistance to intravenous immunoglobulin (IVIG) therapy and the development of coronary artery complications.
Currently, during the treatment of patients in the acute phase of Kawasaki disease, either BNP or NT-proBNP is selectively measured depending on the medical institution. Although some institutions may conduct these tests consecutively, this does not appear to be consistently applied, and the decision may vary depending on the patient. In two large-scale epidemiological studies conducted in Korea during 2012–2014 and 2015–2017, results for natriuretic peptides were missing in a substantial proportion of participants, and among those with available measurements, either BNP or NT-proBNP was measured, but not both [12,13]. This presents a challenge when attempting to include natriuretic peptide levels as candidate variables in predictive analyses of treatment outcomes. In an analysis of the 2012–2014 dataset limited to patients with available NT-proBNP values, NT-proBNP levels failed to predict the development of coronary artery lesions but were identified as a significant predictor of IVIG resistance [6].
This study was designed to explore a method for combining subjects with either BNP or NT-proBNP measurements into a single analytical cohort. Specifically, it was based on the expectation that the z scores derived from log-transformed BNP and NT-proBNP values would each serve as statistically significant predictors with comparable explanatory power.
Methods
The study subjects were comprised of data from two nationwide surveys mentioned previously [12,13]. No additional data collection was conducted for this study. A total of 14,916 patient records were collected in the 2012–2014 survey, and 15,378 records were included in the 2015–2017 survey. Resistance to initial IVIG treatment was defined as the requirement for a second-line therapy. Data from 18,110 individuals with resistance to initial IVIG treatment were pooled for analysis. Of them, 12,537 (69.2%) had pretreatment natriuretic peptide values and were classified as group 1, and the remaining 5,573 (30.8%) were classified as group 2. In group 1, 3,093 subjects had BNP values and 9,444 subjects had NT-proBNP values. No subjects had both BNP and NT-proBNP values.
Resistance to initial IVIG was the outcome of the study. Demographic variables such as age, sex, and body size, as well as laboratory data before the first IVIG administration, were collected as candidate predictor variables. Variables related to coronary artery status were not included in the study dataset.
Natriuretic peptide values were also included as part of the candidate predictor variables. The natural logarithm of these values was computed, and Z scores were subsequently derived for each BNP and NT-proBNP subgroup based on the respective means and SD of the log-transformed values.
Continuous variables were expressed as means ± SD, and categorical variables were presented as frequencies with percentages. Kolmogorov-Smirnov test was performed on natriuretic peptide values and log transformed values. Group comparisons were performed using Student’s t-test and the chi-square test. After reassembling subjects in the BNP and NT-proBNP subgroups through propensity score matching, logistic regression analysis was conducted within each subgroup to identify predictors of resistance to initial IVIG treatment. SPSS version 21 (IBM, USA) was used for statistical analysis, and the statistical significance level was set at P value < 0.050.
Results
The results of the comparison of variables between the two groups and the two subgroups of group 1 are presented in Table 1. In Group 1, the duration before treatment was 5.1 days and the proportion of complete presentation was 66.6%, which were significantly shorter and lower, respectively, than those in Group 2 (5.3 days, P = 0.010; 70.9%, P < 0.001). The resistance to initial IVIG was more frequent in group 1 (15.6%) than in group 2 (11.5%). In Group 1, the white blood cell (WBC) count was 14,000 /μL, neutrophil percentage was 62.9%, albumin level was 3.89 g/dL, and total bilirubin level was 0.63 mg/dL, all of which were significantly lower than those in Group 2 (14,200 /μL, P = 0.005; 63.6%, P = 0.009; 3.92 g/dL, P < 0.001; 0.67 mg/dL, P = 0.013).
BNP: B-type natriuretic peptide; NT-proBNP: N terminal pro-B-type natriuretic peptide; BSA: body surface area; Tx: treatment; inj. Injection; IVIG: intravenous immunoglobulin; WBC: white blood cell; CRP: C-reactive protein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LT-BNP: log transformed BNP; LT-NT-proBNP: log transformed NT-proBNP.
In Group 1, there was no significant difference in the rate of resistance to initial IVIG between the BNP and NT-proBNP subgroups (15.2% vs. 15.8%, P = 0.407). The BNP subgroup had a WBC count of 13,700 /μL, neutrophil percentage of 62.0%, platelet count of 353,800 /μL, alanine aminotransferase (ALT) level of 85.9 U/L, serum sodium level of 135.9 mEq/L, and pyuria frequency of 32.0%, all of which were significantly lower than those in the NT-proBNP subgroup (14,100 /μL, P = 0.001; 63.2%, P = 0.001; 356,100 /μL, P < 0.001; 94.1 U/L, P = 0.008; 136.8 mEq/L, P < 0.001; 36.4%, P < 0.001). In the BNP subgroup, the duration before treatment was 5.3 days, C-reactive protein level was 8.05 mg/dL, total protein level was 6.71 g/dL, and albumin level was 3.92 g/dL, all of which were significantly higher than those in the NT-proBNP subgroup (5.0 days, P < 0.001; 7.67 mg/dL, P = 0.039; 6.55 g/dL, P < 0.001; 3.87 g/dL, P < 0.001).
The values of natriuretic peptides, their log-transformed values, and the corresponding z scores calculated based on their means and SD are presented in Table 1. The distribution of these values is illustrated in Fig. 1.
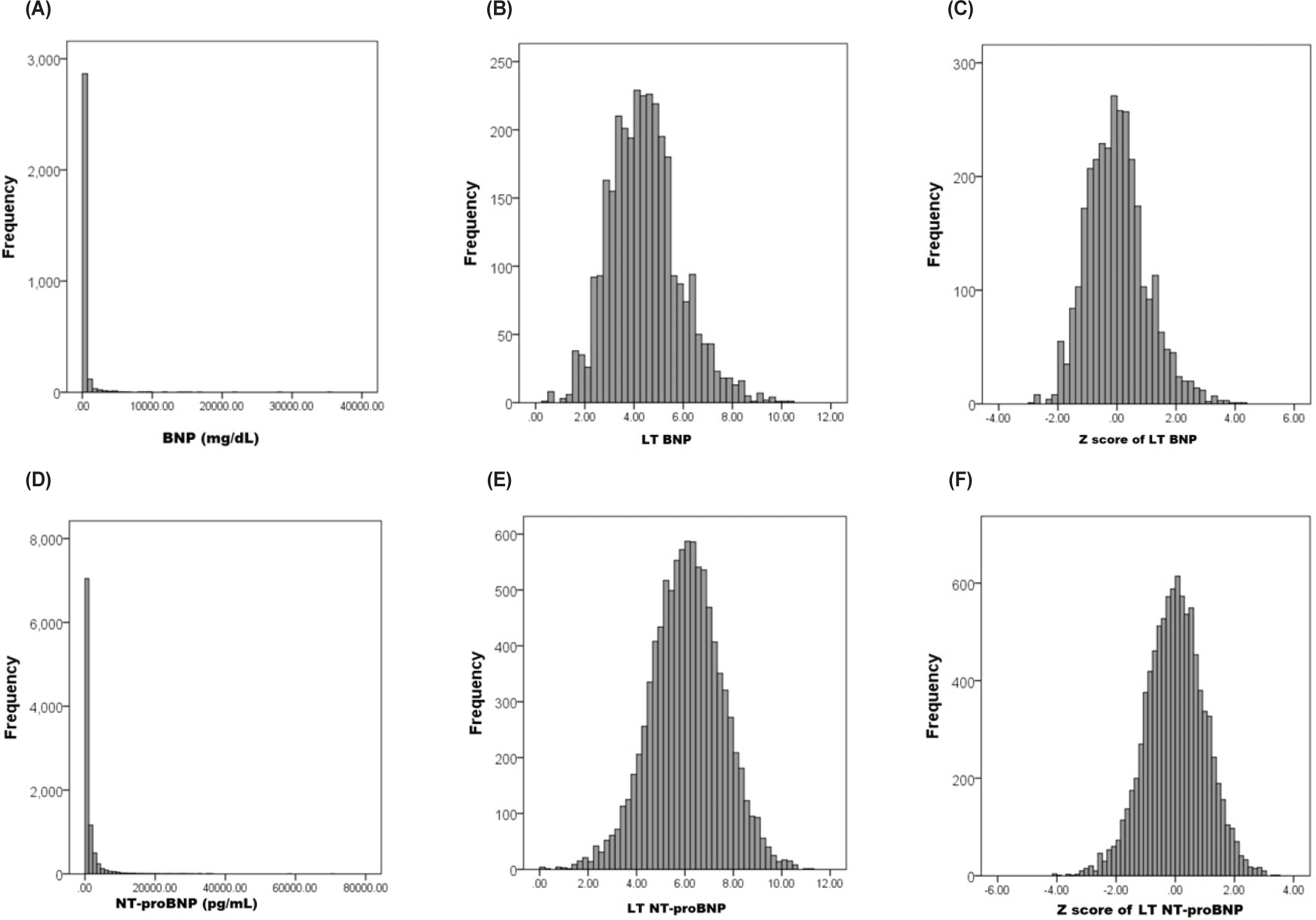
According to the Kolmogorov–Smirnov test, the P values for both the BNP value and the log-transformed BNP value were < 0.001. For NT-proBNP, the P value was also < 0.001, whereas for its log-transformed value, the P value was 0.143.
Among subjects in Group 1, propensity score matching was performed, resulting in the selection of 2,233 matched subjects in each of the BNP and NT-proBNP subgroups. The outcome variable used for matching was the resistance to initial IVIG treatment, and covariates were selected from all variables that showed statistically significant differences between the two subgroups in Table 1.
Univariate and multivariate logistic regression analyses were conducted within each of the reconstituted BNP and NT-proBNP subgroups (Tables 2, 3). In the multivariate analysis, to avoid collinearity, body surface area was selected among body size–related variables, ALT was selected among hepatic aminotransferases, and the z score of the log-transformed value was selected among natriuretic peptides. In the univariate analyses within each subgroup, both BNP and NT-proBNP values were significant predictors, with Nagelkerke R2 values of 0.029 and 0.015, respectively. After log transformation, the values became more comparable (0.024 and 0.025, respectively). The z scores of the log-transformed values of BNP and NT-proBNP remained significant predictors in the multivariate analyses within each subgroup (P = 0.002 and P = 0.026, respectively).
Discussion
In this study, the z scores of log-transformed BNP and NT-proBNP values were significant predictors of resistance to initial IVIG in both univariate and multivariate logistic regression analyses conducted within each respective subgroup. In the univariate analysis, the Nagelkerke R2 value for BNP was 0.029, which decreased to 0.024 after log transformation. For NT-proBNP, the value increased from 0.015 to 0.025 after log transformation, resulting in markedly closer values between the two peptides post-transformation. This convergence of values was maintained after subsequent conversion to z scores. Nagelkerke R2 is an indicator of explanatory power in logistic regression models [14,15]. The value ranges from 0 to 1, with values closer to 1 indicating greater explanatory power. Therefore, the finding that the z scores derived from the log-transformed BNP and NT-proBNP values demonstrated comparable explanatory power suggests the potential feasibility of an integrated use of the two natriuretic peptides. The half-lives of BNP and NT-proBNP are different [16], and few studies investigate how their levels vary according to the day of illness during the acute phase of Kawasaki disease. Therefore, although further investigation is warranted to determine how the timing of measurement may affect their predictive performance, it is noteworthy that the explanatory power of the two natriuretic peptides as predictors became comparable.
Among the total 18,110 patients, 12,537 (69.2%) belonged to group 1, for whom natriuretic peptide results were available. This indicates that the measurement of natriuretic peptides was performed selectively. The proportion of patients with complete presentation was lower in group 1 (66.6%) compared to group 2 (70.9%), while resistance to initial IVIG was more frequent in group 1 (15.6%) than in group 2 (11.5%). Additionally, the mean day of illness at treatment initiation was slightly earlier in group 1 (5.1 days) than in group 2 (5.3 days). These differences suggest that natriuretic peptide testing may have been selectively performed in patients presenting with more severe systemic illness or in those who did not fully meet the diagnostic criteria, thereby prompting more intensive laboratory evaluation. The differences observed in laboratory variables between groups, as well as those between subgroups within group 1, are difficult to interpret consistently. These discrepancies are presumed to reflect variations in institutional clinical practices of the centers where the data were collected. Although propensity score matching was performed to minimize differences between the two subgroups prior to logistic regression analysis, the variation in the composition of variables included in the final multivariate models suggests that residual differences between the subgroups were not fully eliminated.
The primary objective of this study was not to develop the best predictive model for resistance to IVIG, but rather to explore the integration of the two types of natriuretic peptides. We consider that calculating the z scores of each natriuretic peptide within the respective patient groups in which they were measured is likely the only feasible approach to achieve an integrated use of the two natriuretic peptides. Moreover, it is essential that the two patient groups share the same major independent and dependent variables. Therefore, we redefined the two subgroups through propensity score matching. In addition, calculation of z scores requires the use of mean and SD values, and assumes that the underlying data follow a normal distribution. Therefore, for natriuretic peptide values, which often include extreme values, log transformation is essential prior to z score conversion [10]. As demonstrated in this study, log transformation of natriuretic peptide values effectively converted the data into a normal or near-normal distribution (Fig. 1). In the BNP subgroup, although the distribution appeared approximately normal after log transformation, the relatively small sample size limited our ability to confirm normality with certainty. Furthermore, despite the relatively large number of subjects included in this study, those with available natriuretic peptide measurements may not be fully representative of the entire patient cohort. Accordingly, the mean and standard deviation values used for z score calculation in this study are not considered to be universally applicable. In the future, if natriuretic peptide levels can be routinely measured in a large number of patients, it may become possible to establish generalizable reference values for z score transformation.
A key limitation of this study is that measurements of natriuretic peptides were performed selectively across the various participating institutions. Future studies involving larger cohorts with consecutively measured natriuretic peptide levels may yield more robust and generalizable findings. Moreover, such data may help determine the superior peptide, supporting its standardized use across institutions.







