Introduction
Kawasaki disease (KD) is an acute systemic vasculitis of childhood and the leading cause of acquired heart disease among children in developed countries [1]. Timely recognition and treatment are essential because persistent inflammation may result in coronary artery lesions (CALs), leading to long-term cardiac sequelae [2].
Intravenous immunoglobulin (IVIG) at a dose of 2 g/kg remains the standard initial therapy and substantially reduces the risk of coronary complications [1,3]. However, approximately 10%–20% of patients show resistance to initial IVIG, which is associated with a higher incidence of CALs and prolonged hospitalization [1,2,4,5]. Identifying these IVIG-resistant patients early is therefore a critical clinical goal.
To address this, several Japanese risk scoring systems—including the Kobayashi, Egami, and Sano scores—were developed to predict IVIG resistance using routinely available pre-treatment variables such as various clinical and laboratory findings [6–8]. While these models performed well in their derivation cohorts, external validation studies in non-Japanese populations, including Korean and Western cohorts, have shown limited transportability and reduced sensitivity [4,9–13]. These differences likely reflect underlying epidemiologic and biological variability among populations.
Previous Korean studies have been limited by single-center designs and relatively small sample sizes, restricting their generalizability [10,11,13]. Therefore, a nationwide, multicenter validation is essential to ensure reliable estimation of predictive performance across diverse clinical settings and age groups.
The present study analyzed data from a nationwide Korean KD survey encompassing more than 10,000 children to provide the most comprehensive evaluation to date of the Kobayashi, Egami, and Sano scores in this population. In addition, we examined age-stratified predictive performance of Japanese risk models to evaluate their applicability across different age groups.
Methods
This retrospective multicenter cohort study analyzed data from the Korean nationwide Kawasaki Disease (KD) survey conducted between January 2015 and December 2017 [14]. The survey included both residency training hospitals (n = 98) and community hospitals (n = 108), providing comprehensive nationwide coverage. Data collection encompassed demographics, clinical features, treatments, and outcomes, using standardized forms across participating institutions.
We included pediatric patients who were clinically diagnosed with KD and received initial IVIG treatment during the study period. We excluded cases that (1) lacked any variables required to compute the Kobayashi, Egami, or Sano scores, or (2) showed spontaneous defervescence without IVIG administration. The final analytic cohort comprised 10,352 patients.
KD diagnosis followed the 2004 American Heart Association (AHA) scientific statement criteria, including both complete and incomplete KD presentations [3]. IVIG resistance—the primary outcome—was defined as recurrent or persistent fever lasting ≥ 36 hours after completion of the initial IVIG infusion, consistent with the 2004 AHA guideline definition [3]. Because the study period (2015–2017) preceded publication of the 2017 AHA statement, diagnostic and resistance criteria from the 2004 guideline were applied to maintain consistency with contemporaneous Korean national survey methods.
Pre-treatment variables obtained from laboratory data included age, illness days, neutrophil percentage, platelet count, C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and sodium.
The three Japanese IVIG-resistance scores—Kobayashi, Egami, and Sano—were calculated according to their original definitions [6–8]. Each score incorporates routinely available pre-treatment variables, including age, illness days before IVIG, neutrophil percentage, platelet count, CRP, liver enzyme levels, total bilirubin, and serum sodium. High-risk thresholds were defined as ≥ 4 points for the Kobayashi score, ≥ 3 points for the Egami score, and ≥ 2 points for the Sano score, following the criteria established in their respective derivation studies. The detailed components and scoring criteria of each model are summarized in Table 1.
| Risk score | Variable | Criterion (cut-off) | Points | High-risk definition | Ref |
|---|---|---|---|---|---|
| Kobayashi et al. (2006) | Fever days before IVIG | ≤ 4 days | 2 | ≥ 4 points | [7] |
| Age | ≤ 12 months | 1 | |||
| % Neutrophils | ≥ 80% | 2 | |||
| Platelet (× 103 /µL) | ≤ 300 | 1 | |||
| CRP (mg/dL) | ≥ 10 | 1 | |||
| AST (IU/L) | ≥ 100 | 2 | |||
| Sodium (mmol/L) | ≤ 133 | 2 | |||
| Egami et al. (2006) | Age | ≤ 6 months | 1 | ≥ 3 points | [6] |
| Fever days before IVIG | ≤ 4 days | 1 | |||
| ALT (IU/L) | ≥ 80 | 2 | |||
| Platelet (× 103 /µL) | ≤ 300 | 1 | |||
| CRP (mg/dL) | ≥ 8 | 1 | |||
| Sano et al. (2007) | CRP (mg/dL) | ≥ 7 | 1 | ≥ 2 points | [8] |
| Total bilirubin (mg/dL) | ≥ 0.9 | 1 | |||
| AST (IU/L) | ≥ 200 | 1 |
Cases missing any variables necessary for score calculation were excluded from the main analysis.
Continuous variables were summarized as median (interquartile range) and compared between groups using the Mann–Whitney U test, while categorical variables were compared using the x2 or Fisher’s exact test, as appropriate. The predictive performance of each Japanese score was evaluated by calculating sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and the area under the AUC. All statistical analyses were performed using Python (ver. 3.11), and a two-tailed p-value < 0.05 was considered statistically significant.
Results
A total of 15,378 patients with KD were initially identified from the nationwide cohort between 2015 and 2017. After exclusions, 10,352 patients were included in the final analysis. The median age at diagnosis was 32.9 months (interquartile range [IQR], 13.7–46.6), and 57.4% were male. The overall rate of initial IVIG resistance was 16.3% (Table 2).
When applying the three Japanese risk scores, the proportion of patients classified as high risk was 26.3% by the Kobayashi score, 30.0% by the Egami score, and 16.7% by the Sano score.
The predictive performance of each score for IVIG resistance is summarized in Table 2. The Kobayashi and Egami models identified 26.3% and 30.0% of patients as high risk, respectively, yielding sensitivities ranging from 0.46 to 0.47 and specificities from 0.73 to 0.77. The Sano score, which incorporates fewer biochemical variables, achieved the highest specificity (0.87) but the lowest sensitivity (0.33). Consequently, positive predictive values were low (0.25–0.33), whereas NPV remained high (0.87–0.88).
The overall predictive accuracy, expressed as the AUC, was modest for all three scores, ranging from 0.64 to 0.66 (Fig. 1). None of the models reached an AUC above 0.70, indicating limited discriminative ability in this Korean cohort.
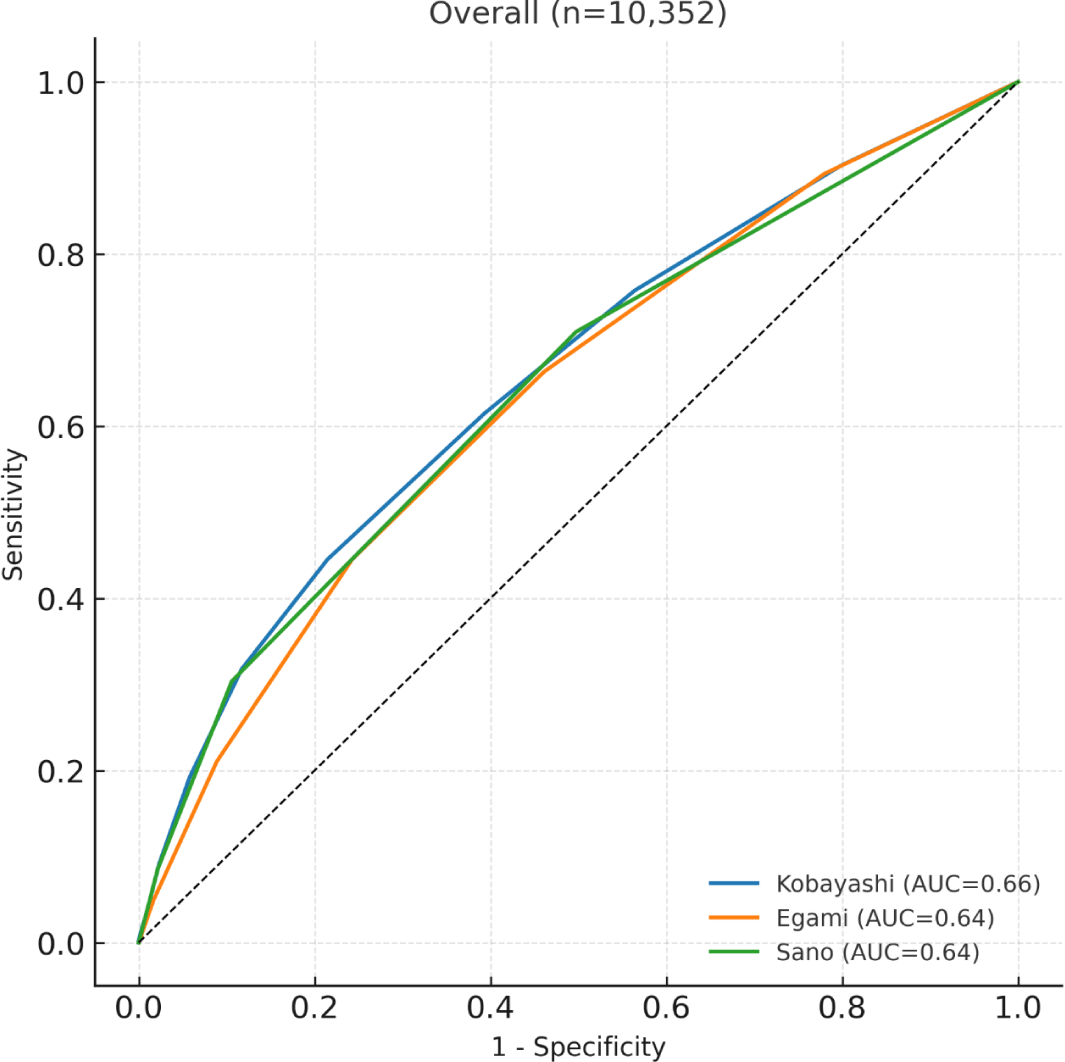
When stratified by age, the three scores showed consistent overall patterns but subtle age-related variations (Table 3).
In infants < 6 months, sensitivities were slightly lower while specificities remained moderate to high, resulting in fair AUCs (0.61–0.66). Both the Kobayashi and Egami scores performed similarly, whereas Sano again exchanged sensitivity for higher specificity (> 0.87).
Among infants aged 6–11 months, discrimination was relatively strongest for all models. Sensitivity peaked in this group—up to 0.53 for Kobayashi—and Egami achieved its highest AUC (0.68). The Sano score maintained its strong specificity (0.90) but continued to show limited sensitivity. Although performance improved slightly in older infants, no model exceeded an AUC of 0.70.
In children 12–59 months, representing two-thirds of the cohort, score performance closely mirrored the overall findings: sensitivities ranged from 0.43 to 0.46, specificities from 0.73 to 0.79, and AUCs from 0.63 to 0.66.
In the ≥ 60 months group, sensitivities of Kobayashi and Egami increased modestly (approximately 0.50), whereas Sano preserved high specificity (0.86). The highest age-specific AUC observed was 0.679 for Kobayashi in this oldest stratum.
Across all age groups, the three Japanese scores consistently demonstrated low sensitivity, high specificity, and only fair overall discrimination, with no subgroup achieving an AUC ≥ 0.70. These trends were clearly reflected in the age-stratified ROC curves, which showed similar overall shapes and consistent separations among the three scores across all age groups (Fig. 2).
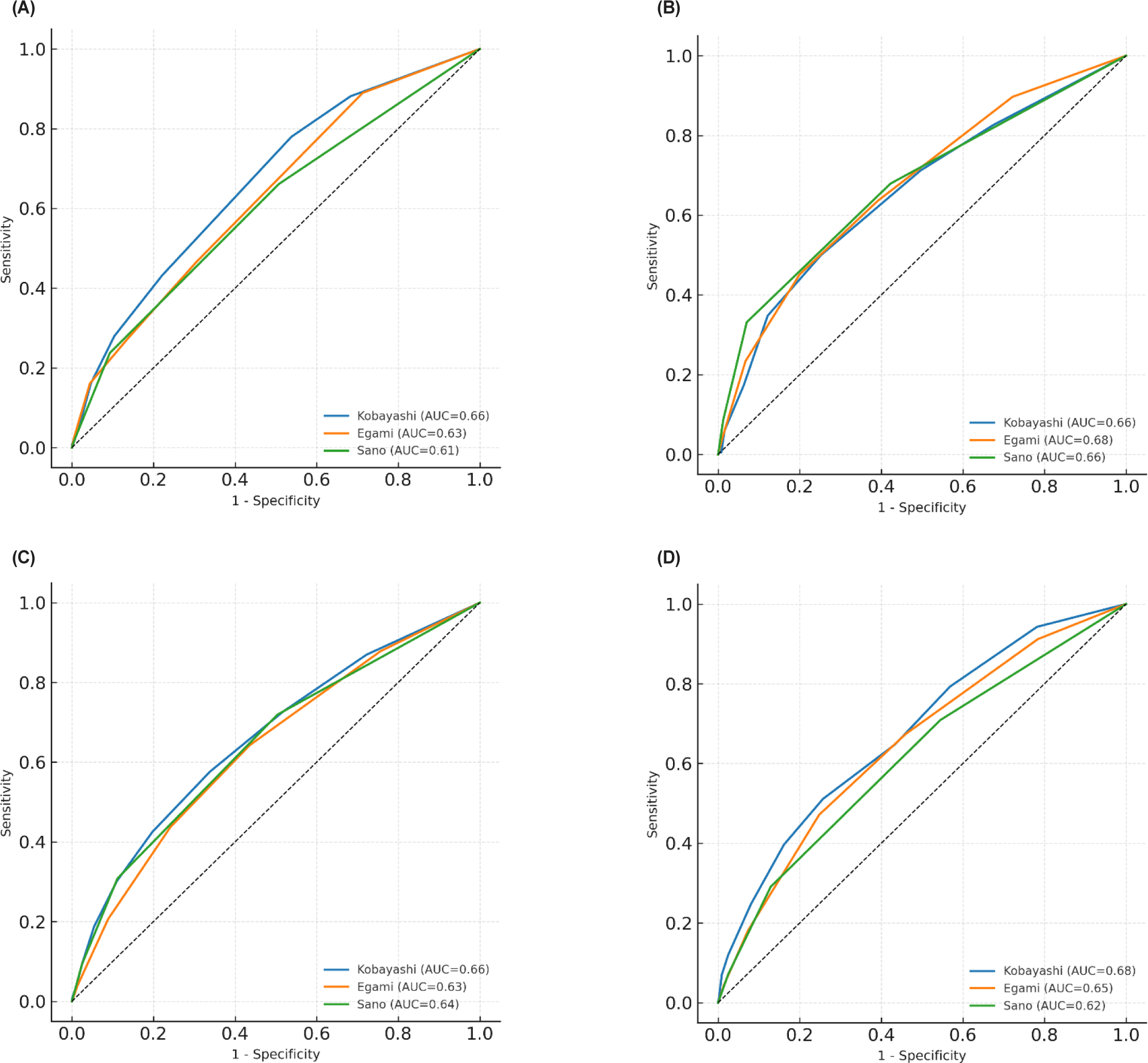
Discussion
This nationwide Korean cohort study evaluated the predictive performance of three Japanese IVIG-resistance risk scores—Kobayashi, Egami, and Sano—in more than 10,000 children with KD. Few studies outside Japan have assessed these models in large, population-based cohorts. In particular, no previous investigation in Korea has applied them to a cohort of this scale, making the present analysis the first to validate Japanese risk scores in a nationwide Korean population. Each of these Japanese scores combines common inflammatory and hepatic parameters such as CRP, AST/ALT, and sodium, but differs in weighting and threshold definitions (Table 1). Across all age strata, the scores demonstrated low sensitivity, high specificity, and only fair discrimination with AUCs ranging from 0.63 to 0.68 across age groups, confirming that these Japanese-derived models are not directly applicable to Korean children.
Our findings are consistent with earlier Korean studies showing that Japanese risk scores maintain high specificity but low sensitivity when applied to local populations. This pattern has been observed across both single-center and multicenter cohorts, as well as in infant-specific analyses, where even modified models incorporating variables such as bilirubin and albumin yielded only marginal improvement in discrimination (AUC < 0.70) [10,11,13,15]. Collectively, the evidence indicates that the limited sensitivity of Japanese scores represents a reproducible trend across Korean cohorts, including our nationwide sample.
Across age groups, the predictive performance of the three Japanese scores was largely similar, with AUCs ranging from 0.61 to 0.68. Slightly higher values were observed in infants aged 6–11 months (Egami AUC 0.68) and in older children aged ≥ 60 months (Kobayashi AUC 0.68), but these differences were modest and did not suggest a consistent age gradient.
In contrast, Shin et al. [13] reported that IVIG-resistant patients were significantly younger than responders, implying a potential influence of age on treatment response. Even after matching for age, however, the Japanese risk scores still showed low sensitivity and high specificity in that study. Together with our findings, this suggests that while age may contribute to the risk of IVIG resistance, it does not substantially improve the predictive ability of existing Japanese models.
The minor AUC variations across age groups in our cohort may instead reflect clinical or laboratory heterogeneity rather than biological differences. Infants with KD often present with incomplete features, diagnostic delay, or atypical inflammatory patterns that could alter laboratory parameters such as CRP, sodium, and bilirubin [7,11,13,16–18]. Although younger age is included as a positive factor in the Kobayashi and Egami scores [6,7], this adjustment did not translate into clearly better discrimination among Korean infants. Although AUCs were relatively higher in infants aged 6–11 months, the absolute discrimination remained modest. Clinically, this suggests that even in younger infants, risk scoring should be complemented by close clinical and laboratory monitoring rather than used as a standalone predictor.
While we did not directly analyze individual predictors, prior work in Korean cohorts provides insight into potential sources of reduced model sensitivity. Shin et al. demonstrated that sodium and bilirubin cutoffs derived from Japanese populations were suboptimal in Korean children [13]. Lim et al. similarly proposed that population-level shifts in laboratory distributions—specifically lower CRP and bilirubin and higher sodium—may weaken risk separation at fixed cutoffs [11]. Such inter-population heterogeneity likely reflects both genetic and environmental factors influencing inflammatory response. At the same time, improvements in early diagnosis and standardized IVIG administration in Korea may have narrowed the laboratory gap between responders and non-responders, further diminishing discrimination [19,20] These inter-model differences likely stem from distinct weighting of hepatic versus inflammatory variables. For example, the Sano model prioritizes hepatic markers (AST and bilirubin), whereas the Kobayashi and Egami scores emphasize inflammatory burden and hyponatremia, leading to differences in performance across age groups and populations.
Among the three Japanese scores, the Sano model consistently demonstrated the highest specificity across diverse external validations. In a Korean single-center study by Park et al., specificity reached 90% [15]; in a large French multiethnic cohort, 86% [12]; and in a recent nationwide Korean infant cohort, 95.9% [11]. This consistently high specificity likely reflects the stricter biochemical thresholds of the Sano model, particularly elevated AST and bilirubin levels.
Mechanistically, the observed variability in score performance across populations can be explained by several overlapping factors. Laboratory distributions differ between Japanese and Korean cohorts, with lower CRP and bilirubin and higher sodium levels in Korean children attenuating risk separation at the original Japanese cutoffs [11–13]. Differences in patient composition, including a higher proportion of incomplete KD and younger infants who often show delayed or atypical inflammatory responses, may also weaken the predictive contribution of laboratory-based criteria [11,14]. In addition, earlier diagnosis and the standardized use of a single 2 g/kg IVIG regimen in Korea likely reduce pre-treatment laboratory extremes, narrowing the contrast between responders and non-responders [12,14]. Finally, model calibration issues contribute to these discrepancies: Japanese scores were derived from cohorts with different baseline prevalences and treatment protocols (for example, 1 g/kg for two consecutive days in the original Kobayashi study [7]), so their operating thresholds may shift when applied to contemporary Korean practice. Together, these factors underscore the need for local recalibration and context-specific model development [4,11,12]. Consistent patterns have been observed in other Asian and non-Asian populations. Across cohorts from Taiwan, Europe, and non-Asian countries, the Japanese risk scores showed uniformly low sensitivity but preserved specificity (AUCs mostly < 0.70). These findings indicate that the predictive limitations of the Japanese models are not confined to Korean children but represent a broader issue of population-level applicability. Variations in genetic background, baseline laboratory profiles, IVIG formulation, and treatment timing have all been proposed as potential contributors to this inconsistency [4,9,12].
European studies further emphasized that differences in disease presentation and laboratory ranges necessitate region-specific recalibration of existing scores and locally validated decision tools [9,21,22]. Additional variability may arise from disparities in echocardiographic measurement standards and inflammatory marker distributions [12,23]. Genetic susceptibility loci such as inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) and Fc Gamma Receptor IIa (FCGR2A) also vary across populations, suggesting that biological heterogeneity contributes to divergent model performance [24,25]. Together, these factors highlight that epidemiologic, clinical, and genetic diversity across populations limits the generalizability of Japanese IVIG-resistance scores and underscores the need for recalibrated or newly developed models tailored to local characteristics.
Clinically, these findings emphasize that current scoring systems are best interpreted as exclusionary rather than confirmatory tools. Their high specificity but low sensitivity means that a low score increases the likelihood of IVIG response, but does not guarantee it, while a high score does not necessarily indicate resistance. For clinicians in Korea, early recognition of potential nonresponse should therefore rely not solely on static risk scores but on dynamic reassessment of clinical and laboratory evolution. Integrating prediction models into such longitudinal clinical judgment, rather than treating them as fixed thresholds, may improve individualized management decisions.
This study has several limitations. Because of its retrospective design, residual confounding and data incompleteness are possible. Coronary outcomes were not evaluated, so the link between IVIG resistance and long-term cardiac sequelae remains to be clarified. We assessed the Japanese models using their original cutoffs and did not attempt recalibration, which should be the focus of future research. Additionally, because the dataset covers 2015–2017, more recent clinical or laboratory trends may not be fully reflected. Despite these limitations, the large sample size, national coverage, and standardized data collection provide the most comprehensive validation to date of Japanese IVIG-resistance scores in Korean children.
In conclusion, Japanese risk scores for IVIG resistance demonstrate consistent but suboptimal performance when applied to Korean children, with low sensitivity, high specificity, and only fair overall discrimination. These findings clearly demonstrate that direct application of Japanese IVIG-resistance risk scores is suboptimal for Korean children with KD. The results highlight the urgent need to develop a Korean-specific risk model to improve early identification and tailored management of IVIG-resistant patients.







