Introduction
Kawasaki Disease (KD) is a systemic vasculitis that primarily affects children under five years of age, with coronary artery involvement as its most significant complication. Coronary artery aneurysms or ectasia develop in approximately 15% to 25% of untreated KD, and this can lead to myocardial infarction or ischemic heart disease. This report details a case of young KD patient with coronary thrombosis successfully treated with tissue plasminogen activator (tPA), in line with guidelines by the American Heart Association (AHA) and Japanese Circulation Society (JCS) recommendations [1,2].
Case
A previously healthy 23-month-old male presented with lethargy, nausea, and poor oral intake, followed by cyanosis and general tonic-clonic seizure after vomiting. This led him to visit a nearby hospital’s emergency room, where pulseless electrical activity was observed on an ECG, necessitating cardiopulmonary resuscitation (CPR). Defibrillation was performed for ventricular fibrillation, and spontaneous circulation was restored after a total of 10 minutes of CPR. The patient was subsequently transferred to our hospital.
Retrospectively, he had suffered from two episodes of fever. Approximately three weeks prior, he had presented with a fever of six days’ duration with a maculopapular rash. A few days after this fever subsided, a second febrile episode due to an influenza infection lasted five days and then subsided. His temperatures were normal during the past week before the day of cardiopulmonary arrest.
Upon arrival at our facility, his initial vital signs had relatively stabilized. His blood pressure was 91/47 and heart rate was 158 without fever. We put the child under normothermia and conducted further evaluation. Elevated myocardial enzyme levels including initial troponin T of 1318 (N: < 14 pg/mL), CK 2912 (N: 44–245 IU/L), CK-MB 1669 (N: 0.0–5.0 ng/mL), NTproBNP 607 (N: 39–675 pg/mL) upon ER admission raised suspicions of myocarditis or other congenital heart defects such as anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), which were considered in the differential diagnosis. Other potential etiologies, such as seizure due to neurological disorder or respiratory arrest from aspiration, were ruled out.
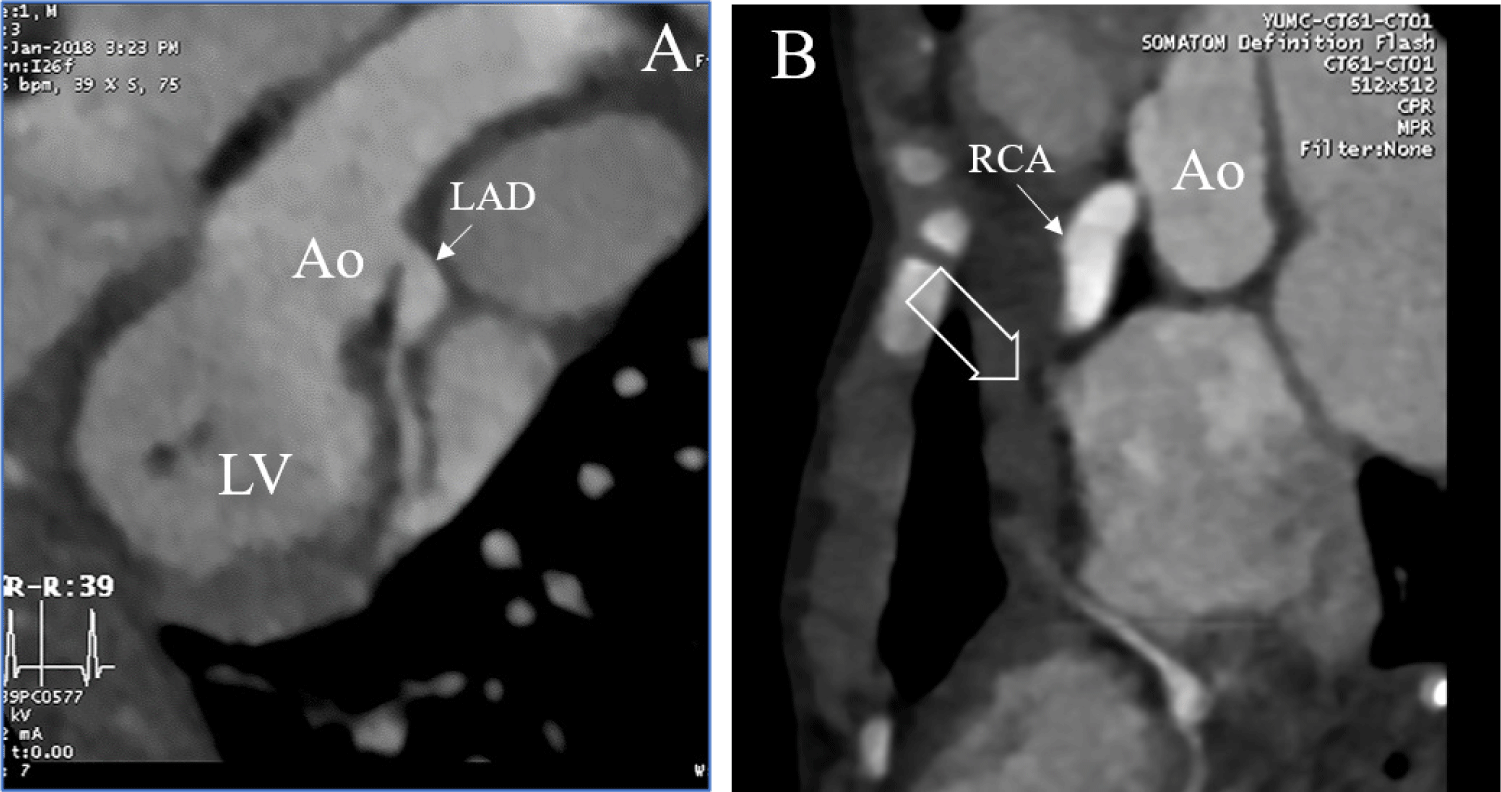
ST elevation in the inferior leads with ST depression at the lateral leads were noted at the initial electrocardiogram. Echocardiography (Fig. 1) revealed preserved left ventricular systolic function without the use of inotropics; however, a large coronary artery aneurysm (maximum diameter of 8 mm, Z-score: 8.6) in the left anterior descending artery and severe stenosis at the right coronary ostium with another large coronary aneurysm (maximum diameter of 8.2 mm, Z-score: 7.7) were observed, without distal blood flow. Here, congenital heart defects from the differential diagnosis were excluded. This correlated with the computed tomography (CT) results: A fusiform aneurysm from the proximal to the mid-right coronary artery (RCA) with thrombotic occlusion observed at the proximal RCA with severe stenosis at the RCA ostium, with another fusiform aneurysm from the proximal to the mid-left anterior descending (LAD) with subendocardial low attenuation at the RCA territory (Fig. 2). Other imaging studies, including brain magnetic resonance imaging (MRI) and abdominal ultrasonogram, did not show any aneurysms in other parts of the body.
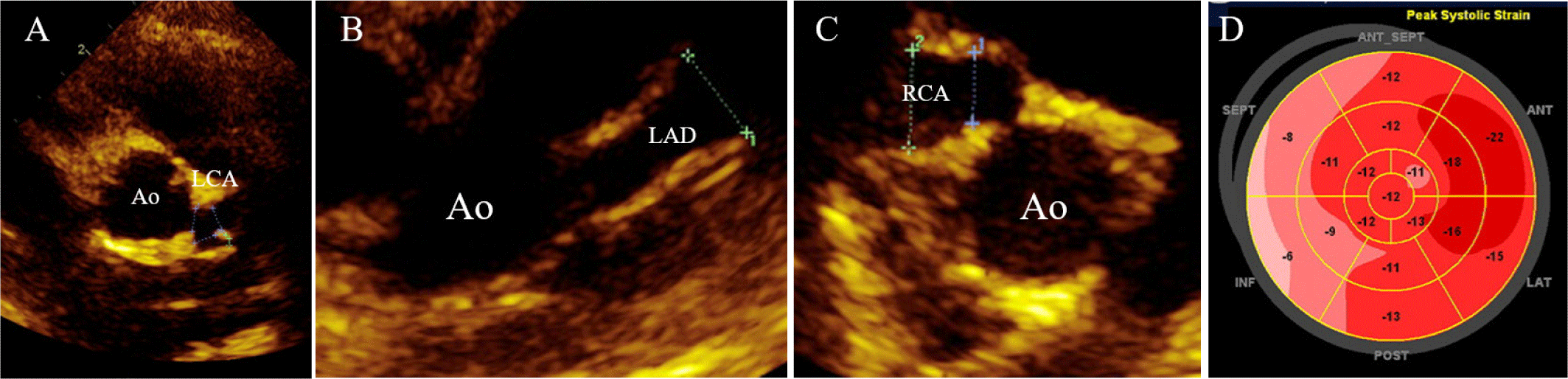
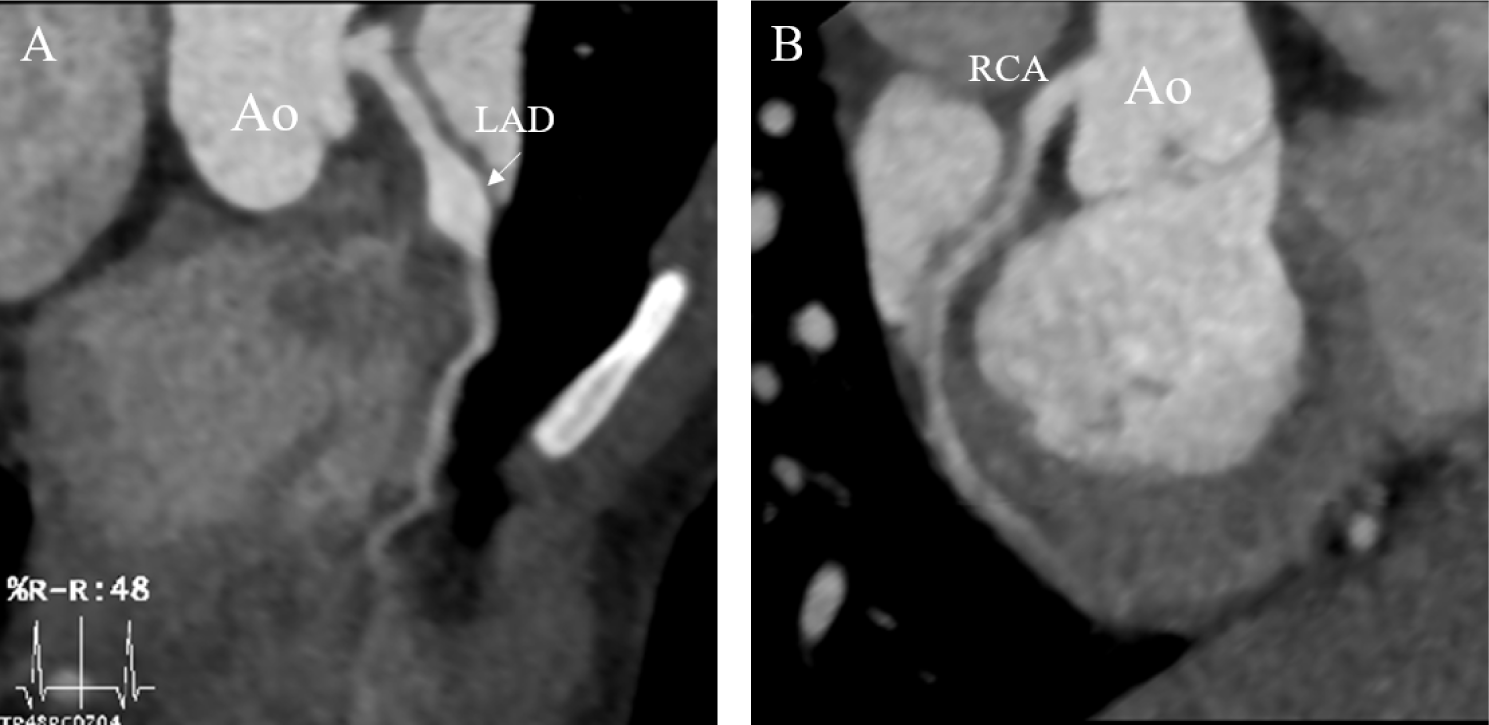
Further history taking revealed that the fever episode three weeks prior to admission met the diagnostic criteria for KD retrospectively. The patient also has showed the following symptoms with the six-day fever: 1) bilateral conjunctival injection, 2) red lips and tongue, 3) generalized rash, and 4) redness and swelling of the hands. Considering the occurrence of myocardial infarction during the acute/subacute phase of KD and the infantile-onset with the patient’s body weight of 10 kg at the time of diagnosis, Alteplase was administered at a dose of 0.5 mg/kg/hr as a continuous six-hour infusion, in alignment with recommendations from the JCS guideline for pediatric coronary thrombolysis [2]. During tPA infusion, careful monitoring was conducted to detect bleeding complications. Other than mild gastrointestinal bleeding, no significant complications were observed. Coronary reperfusion in the right coronary artery was confirmed through cardiac CT one month later. Serial echocardiography and cardiac CT afterward, 6 months, two-year, and five-year follow-up (Fig. 3) revealed no recurrence of coronary thrombosis. The coronary artery aneurysm also showed regression, with its size decreasing from the largest diameter of the RCA measuring 8.5 mm (z = 7.57) initially to 4.2 mm (z = 3.11) at the five-year follow-up and LAD 8 mm (z = 8.60) to 7.0 mm (z = 6.52). The patient is currently under dual antiplatelet therapy and anticoagulant without any bleeding tendencies, keeping an INR of 2.0–2.5 to manage bilateral coronary artery aneurysms and stenosis.
Discussion
Early reperfusion therapy via primary percutaneous coronary intervention (PCI) or thrombolysis using tPA is both established treatments in acute myocardial infarction due to acute coronary thrombosis according to the JCS guideline. However, there are no comparative studies between these two reperfusion methods. Although primary PCI is ideal in adolescents with suitable vessel size for catheter intervention, thrombolytic therapy is recommended in cases of acute coronary syndrome (ACS) in small children complicated by KD. This case report supports tPA’s role as an effective intervention for acute coronary thrombosis in KD, especially in infants.
Although there is scarce data on tPA use for ACS in pediatric KD, adult studies in ACS and a few pediatric KD case series provide evidence for tPA efficacy [3]. Possible side effects of tPA include increased risk of bleeding and distal thromboembolism. During tPA infusion, low-dose aspirin and low-dose IV heparin (10 U/kg/hour), along with gastrointestinal protective therapy, should be administered concurrently. To minimize the risk of bleeding, careful monitoring is required to maintain fibrinogen levels (> 100 mg/dL) and platelet counts (> 50,000 /mm3) [4]. Those with current intracranial hemorrhage or other active internal bleeding or recent history of stroke or head trauma are contraindicated. Given its noninvasiveness, successful use of tPA in this case demonstrates its potential in managing coronary thrombosis in pediatric KD patients who are not feasible for catheter interventions. This case highlights the importance of incorporating thrombolytic agents in KD treatment protocols, especially for patients at high risk of coronary artery occlusion.







