Introduction
High-dose intravenous immunoglobulin (IVIG) is the standard first-line therapy for Kawasaki disease (KD) to control inflammation and reduce the risk of coronary artery lesions (CALs). However, 10–20% of patients fail to respond to IVIG therapy. Many studies have shown that patients with KD who have a persistent or recrudescent fever after primary IVIG therapy have an increased risk of developing CALs. Additional treatment for IVIG-resistant KD patients mainly consists of 2nd dose of IVIG, corticosteroids, and infliximab [1].
Procalcitonin (PCT), the prehormone of calcitonin, is released in response to proinflammatory stimuli, particularly bacteria-associated mediators. The relation between PCT and IVIG-resistant KD was also reported. KD patients with high PCT level had a significantly higher incidence of IVIG-resistant disease [2]. PCT is mainly produced in the liver and monocytes, and its secretion is modulated by cytokines such as TNF-α and interleukins [3]. So, TNF-α blockade, infliximab may be useful in the case of IVIG-resistant KD with high PCT level.
Case
The 17-month-old boy was transferred to our hospital with five days of fever and elevated C- reactive protein (CRP). He presented with remittent fever, with temperatures rising up to 39°C. The physical examination showed oral and pharyngeal erythema, red lips, and a skin rash on the trunk and foot. His laboratory results on admission showed a white blood cell count of 13,270 /mm3, with 41.4% segmented leukocytes and 44.2% lymphocytes. Additionally, his hemoglobin level was 9.8 g/dL, platelet count was 433,000 /mm3, C-reactive protein was 10.7 mg/dL, and pro-brain natriuretic peptide was 2,106 pg/mL. Other laboratory findings are presented in Table 1. Blood and urine cultures yielded no growth.
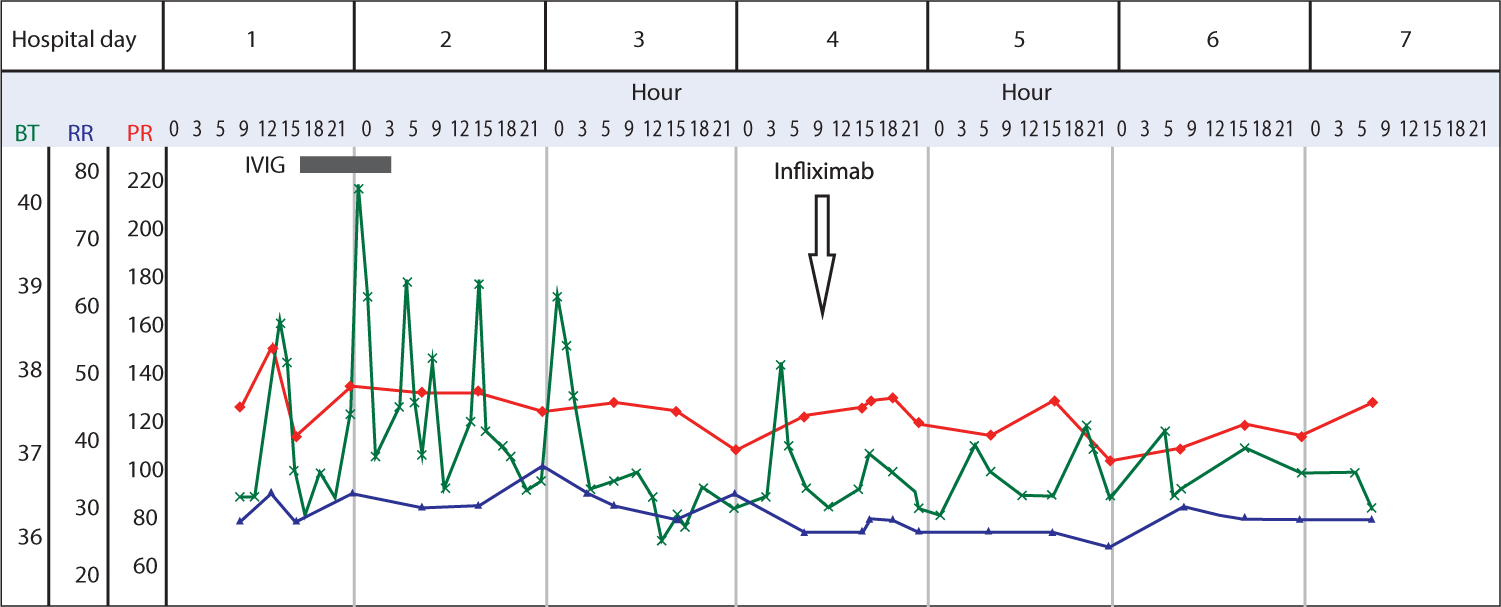
He received a single dose of IVIG (2 g/kg) and a moderate-dose aspirin (50 mg/kg/day). However, a recrudescent fever occurred 36 hours after the completion of IVIG. On the third day of hospitalization, the PCT level was elevated, measuring 39.5 ng/mL, and the CRP was also increased to 16.6 mg/dL. Infliximab (8 mg/kg) was administered intravenously. Following this, the fever subsided, and by the fourth day of hospitalization, both CRP and PCT levels had significantly decreased to 7.1 mg/dL and 9.7 ng/mL, respectively, along with clinical improvement (Fig. 1, Table 1). Fingertip desquamation appeared approximately two weeks after the onset of the fever.
On the first day of hospitalization, an echocardiography showed normal ventricular function, along with slight dilation observed in the left coronary artery. The specific measurements were as follows: the right coronary artery measured 2.3mm (z score: 1.7), the left main coronary artery measured 2.6 mm (z score: 2.07), the left anterior descending coronary artery measured 2.3 mm (z score: 2.06). There was trivial mitral valve regurgitation and a scanty amount of pericardial effusion.
He was discharged with low-dose aspirin (5 mg/kg/day). On clinic follow-up on two months after symptom onset, echocardiography showed normal-sized coronary arteries.
Discussion
Recent studies have revealed that PCT can serve as a marker for bacterial infection and sepsis [4]. The correlation between PCT level and IVIG-resistant KD has been reported. A clinical trial conducted at Boston Children’s Hospital in the USA indicated that a serum PCT level ≥ 0.5 ng/mL was associated with non-responsiveness to IVIG. Moreover, a PCT concentration of ≥ 4.3 ng/mL exhibited the highest sensitivity and specificity values for predicting IVIG non-responsiveness [2].
However, Shao et al. [5] recruited 530 KD patients and found that serum PCT levels were significantly higher in IVIG non-responders. Nevertheless, PCT may not be suitable as an independent predictive factor for both initial and repeated IVIG resistance in KD.
In our case, the patient was administered IVIG and a moderate-dose aspirin. However, there was recrudescent fever. Since IVIG resistance is likely to manifest with high CRP and PCT levels, and a multicenter study conducted in Korea found that the benefits of infliximab are more significant when administered earlier in the patient’s course [6], we have decided to administer infliximab as a second-line treatment. We administered the maximum dose of infliximab, 8 mg/kg (1 vial, 100 mg), instead of the standard 5 mg/kg. This decision was based on the KIDCARE trial [7], where the dose of infliximab (10 mg/kg) was chosen based on pharmacokinetic modeling, which suggested a decrease in the concentration of the drug in the tissue compartment when given after IVIG administration [8]. After infliximab administration, the fever subsided, and CRP and PCT levels decreased dramatically.
Although there have been no previous reports of tuberculosis reactivation following infliximab treatment in a patient with KD, we conducted a tuberculin skin test on this patient before initiating infliximab therapy, and the result was negative.
PCT is mainly produced in the liver and monocytes, with its secretion being modulated by lipopolysaccharides and cytokines such as TNF-α. Therefore, infliximab (TNF-α blockade) may be useful for KD patients with high PCT level. This case report suggests that the treatment of infliximab may be useful for IVIG-resistant KD with high PCT level.
However, another study exhibited contradictory findings. They reported that high procalcitonin levels or low sodium levels independently predicted the resistance to infliximab therapy in KD patients refractory to IVIG treatment. The study concluded that PCT might serve as an effective biomarker for predicting infliximab resistance in severe KD patients who do not respond to IVIG treatment [9]. Therefore, further study is needed.
In conclusion, we report a case of IVIG-resistant KD with high PCT level who was successfully treated with infliximab. This case report suggests that the treatment of infliximab may be useful for IVIG-resistant KD with high PCT level.







