Introduction
The principal initial treatment for Kawasaki disease (KD) consists of intravenous immunoglobulin (IVIG) and oral acetylsalicylic acid, and most KD patients respond to the initial treatment. However some patients show resistance to the initial treatment, and they are considered as having IVIG-resistant KD or refractory KD. Refractory KD is defined as KD resistant to initial IVIG therapy, that shows persistent or recrudescent fever at least 36 hours after completion of initial IVIG therapy [1]. The proportion of refractory KD is reported to be 10%−20% according to studies [1-3]. The risk of coronary arterial aneurysm (CAA) is increased in refractory KD, so early identification and appropriate treatment of refractory KD are essential to prevent CAA. Recommended treatment modalities for refractory KD are additional IVIG, corticosteroids, infliximab, immunosuppressants, plasmapheresis, etc.
We have been performing nationwide surveys on KD triennially since 1991 to know the epidemiologic features of KD in South Korea. In this study, we analyzed the data of Korean nationwide surveys on KD to investigate the epidemiology and clinical outcomes of refractory KD in South Korea, and to see whether there were any changes in the proportion and treatment modality of refractory KD over time.
Methods
We reviewed the data of executive three triennial Korean nationwide surveys on KD from 2009 to 2017 [4-6], and selected patients with refractory KD who had persistent or recrudescent fever at least 36 hours after completion of initial IVIG therapy. Then we analyzed the data of patients with refractory KD to know the proportion of refractory KD, treatment modalities for refractory KD, the proportion of incomplete KD among patients with refractory KD, and the incidence rates of CAA in refractory KD. The comparison of the annual proportions of refractory KD was performed using the Mack-Wolfe test, and comparisons of proportions of incomplete KD and incidence rates of CAA were performed using the Chi-square test.
Results
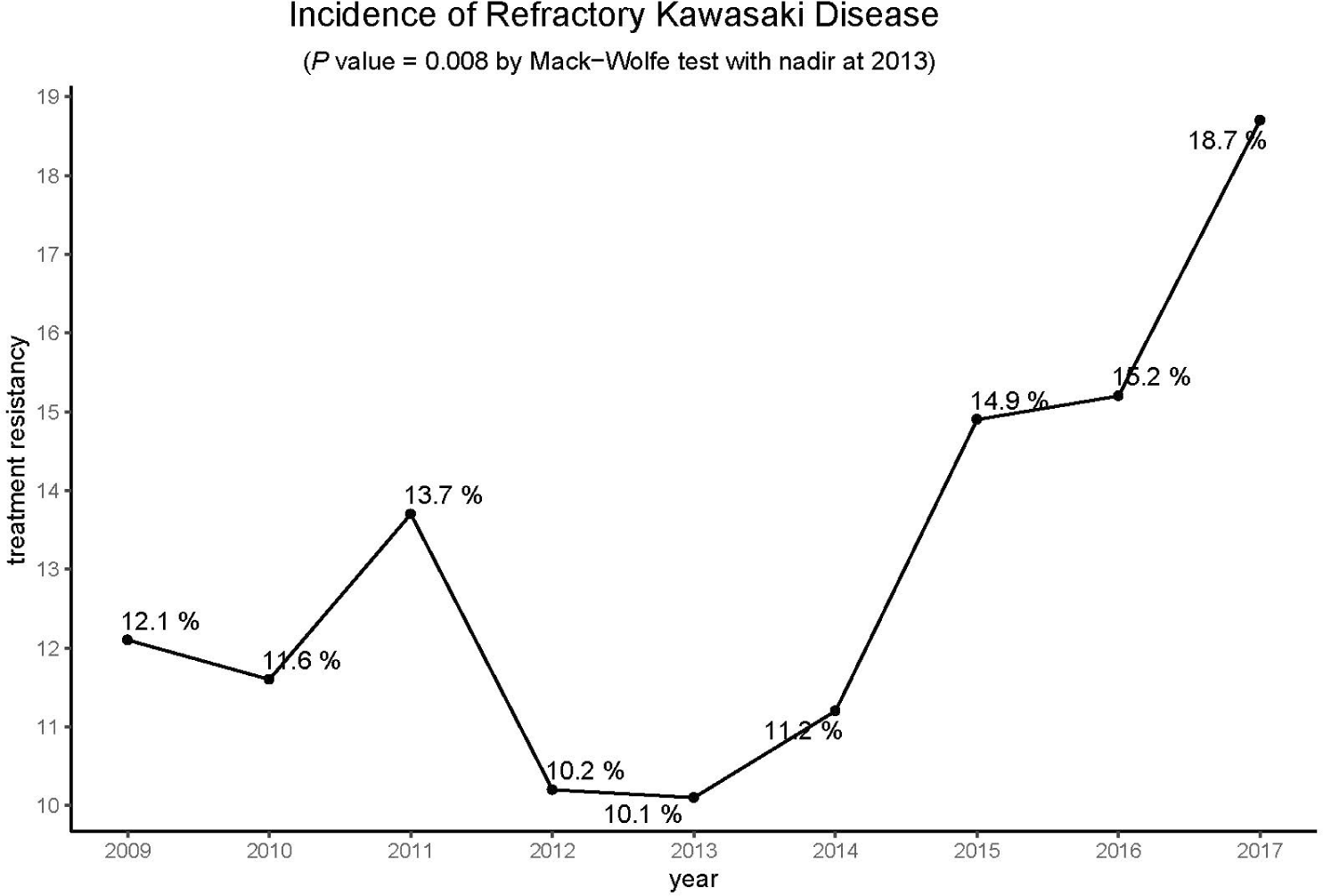
Among a total of 42,705 KD patients from 2009 through 2017, 5,581 (13.1%) had refractory KD. The numbers of all KD patients and patients with refractory KD and the annual proportions of refractory KD from 2009 through 2017 are summarized in Table 1. The annual proportions of refractory KD were between 10.1% and 18.7% from 2009 through 2017. The annual proportions of refractory KD showed significant changes with the nadir in 2013 and increased proportions in 2015−2017 (P < 0.05) (Fig. 1). The proportions of incomplete KD among patients with refractory KD were as follows; 42.2% in the 2009−2011 period, 32.8% in the 2012−2014 period, and 44.9% in the 2015−2017 period. It showed no significant difference over time.
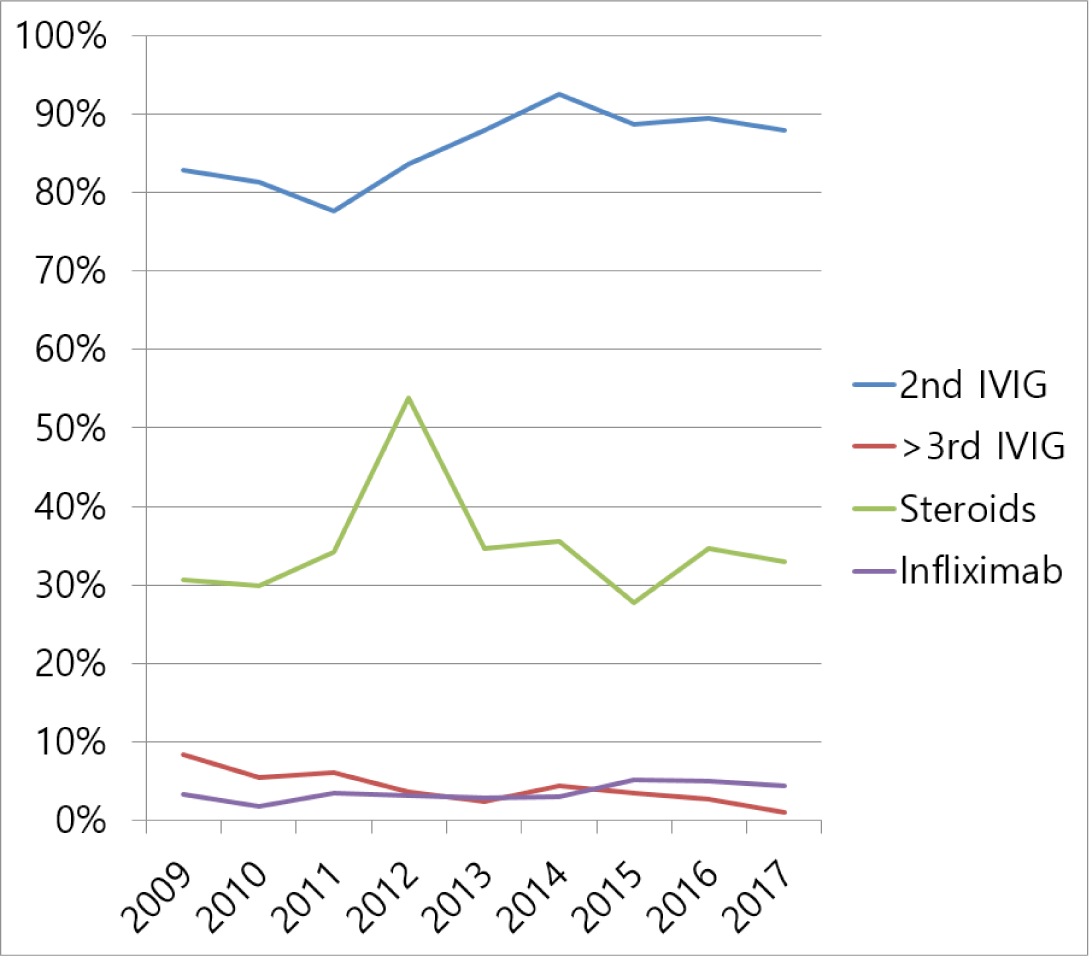
Treatment modalities for refractory KD were as follows. Second IVIG was used in 86.2% of patients with refractory KD, third or more IVIG in 3.8%, corticosteroids [intravenous (IV) methylprednisolone, oral prednisolone, IV or oral dexamethasone, IV hydrocortisone] in 34.4%, infliximab in 3.7%, methotrexate in 1.0%, plasmapheresis in 0.1%, and cyclosporine in 0.02%. The use of third or more IVIG showed a decreasing trend with time, and the use of corticosteroids and infliximab showed an increasing trend with time (Fig. 2).
The overall incidence rate of CAA in patients with refractory KD was 5.5% from 2009 through 2017, which was significantly higher than that in all KD patients from 2009 through 2017 (5.5% vs 1.8%; P < 0.05). The annual incidence rates of CAA in patients with refractory KD were as follows; 7.6% in 2009, 6.1% in 2010, 5.0% in 2011, 5.5% in 2012, 6.8% in 2013, 5.2% in 2014, 5.3% in 2015, 5.2% in 2016, and 4.2% in 2017 (Table 2). They showed no significant difference over time. Mortality in patients with refractory KD was zero from 2009 through 2017 (Five KD patients died during the study period, but they were not diagnosed as having refractory KD).
| Year | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|
| Incidence rate of CAA (%) | 7.6 | 6.1 | 5.0 | 5.5 | 6.8 | 5.2 | 5.3 | 5.2 | 4.2 |
Discussion
Approximately 10% to 20% of KD patients develop persistent or recrudescent fever at least 36 hours after completion of initial IVIG therapy plus oral acetylsalicylic acid, and they are diagnosed as having IVIG resistance or refractory KD. It is postulated that host genetic factors, such as polymorphisms in the Fc gamma receptors, play a role in both the response and resistance to IVIG [1]. Persistent or recrudescent fever after initial therapy is assumed to be caused by ongoing vasculitis, if other causes of fever are not excluded, such as adverse reaction to IVIG or concomitant infections. Therefore, the risk of significant complications or sequelae, including CAA, is markedly increased in refractory KD. In one study including 378 KD patients, patients who remained febrile had an almost nine-fold increased risk of developing CAA compared to those who responded to initial IVIG (12.2% vs 1.4%) [7]. Thus, refractory KD has been a significant challenge to physicians who treat KD patients. Although many studies have focused on the prediction and treatment of refractory KD, there have been few studies on the epidemiology and clinical outcomes of refractory KD. Three scoring systems for the prediction of refractory KD were developed in Japan [8-10], although their usefulness remains debatable in other countries. Recently, prediction models for IVIG resistance were developed based on machine learning algorithms [11-14]. Recommended treatments for refractory KD include additional IVIG, corticosteroids, infliximab (monoclonal antibody to tumor necrosis factor-alpha), cyclosporine, cytotoxic agents, plasmapheresis, etc.
Moffett et al. reported the epidemiology of IVIG-resistant KD in the USA from January 2004 to June 2012 [2]. According to the study, the overall incidence of IVIG resistance in 33 hospitals was 16.3% (range 8%−26.8%). The study revealed that the incidences of IVIG-resistant Kawasaki disease (KD) did not show significant variations over time. Furthermore, no significant differences were observed in the age and sex distributions of KD patients between hospitals with high and low IVIG resistance rates. However, it was noted that African-American KD patients were more prevalent in hospitals with high IVIG resistance rates. The authors postulated that the difference in incidences of IVIG resistance among hospitals might occur due to variations in the diagnosis or treatment of KD patients [2].
Tremoulet et al. reported the epidemiology of IVIG-resistant KD in San Diego, USA [3]. From 1998 through 2005, the annual percentage of IVIG-resistant KD was from 9.8% to 20%. In 2006, however, there was an extraordinary increase in the incidence of IVIG resistance to 38.3% (P = 0.009). Despite this increase, there was no significant difference in clinical presentations between KD patients in 2006 and those in 2005, when the incidence of IVIG resistance was 11.1%. The study could not find any association between IVIG resistance and specific brands or lots of IVIG. Investigation of IVIG characteristics, such as manufacturing methods, release and shipment, stability, IgG content, anti-complement activity, and trypsin/chymotrypsin exposure time, did not reveal any significant changes. The authors found that IVIG resistance was associated with earlier diagnosis of KD, higher percentage of the band form among white blood cells, higher C-reactive protein (CRP), higher alanine aminotransferase, higher gamma-glutamyltranspeptidase, lower hemoglobin, and lower platelet count. Furthermore, IVIG-resistant KD patients had a higher incidence of CAA compared to IVIG-responsive patients (15% vs 3%; P = 0.0008) [3].
Son et al. conducted a study in the USA to assess the treatment outcomes of KD [15]. The study included 4811 KD patients from 27 pediatric hospitals from 2001 to 2006. The re-admission rate due to relapse of fever was 7.3%, and there were no significant changes over the study period. It was higher in KD patients less than 1 year old compared to those over 1 year old (9.6% vs 6.8%; P = 0.005). Re-treatment included a second or more IVIG (14.8%), IV methylprednisolone (5.8%), oral prednisone (2.8%), and infliximab (1.0%). The use of infliximab increased from 0% in 2001 to 2.3% in 2006 [15].
Dionne et al. noticed the association of IVIG resistance with concomitant infections in KD patients [16]. They analyzed the data of 154 KD patients in a tertiary university hospital in Canada between 2008 and 2016 and found that 59 (38%) had concomitant infections. These included 20 viral infections (19 upper respiratory tract infection, 2 viral gastroenteritis, 3 cytomegalovirus infection) and 39 bacterial infections (13 group A streptococcal pharyngitis, 8 pneumonia, 7 otitis media, 4 bacterial lymphadenitis, 2 mycoplasma infection, 1 pyelonephritis, 1 perforated appendicitis). The authors found that KD patients with concomitant infections were more likely to have fever 48 hours after initial IVIG treatment (36% vs 20%; P = 0.05), and more likely to be treated with second IVIG (33% vs 18%; P = 0.04). KD patients with concomitant infections had higher CRP at the time of diagnosis (14.8 vs 11.2 mg/dL; P = 0.04) and 48 hours after initial IVIG treatment (111 vs 59 mg/dL; P = 0.003). The incidences of coronary arterial complication (Z-score > 2.5) showed no significant difference between KD patients with and without concomitant infections (36% vs 39%; P = 0.68). The authors concluded that concomitant infections might be the cause of persistent fever in KD patients. They suggested that the decision on second-line treatment in KD patients with concomitant infections should not only be based on persistent fever, and coronary arterial dimensions may be a more useful indication for second-line treatment in these patients. In addition, they proposed prospective studies to better refine which KD patients truly require additional therapies [16].
In this study, we analyzed the data from Korean nationwide surveys on KD to investigate the epidemiology and clinical outcomes of refractory KD in South Korea, and to see whether there were any changes in the proportions and treatment modalities of refractory KD over time. However, there are some limitations in this study. We could not analyze the whole data of Korean nationwide surveys on KD since 1991, and could not compare clinical features between refractory KD patients and IVIG-responsive KD patients due to insufficient data. Nonetheless, we were able to gain insights into the epidemiology and clinical outcomes of refractory KD in recent years in South Korea. The annual proportions of refractory KD showed increasing trend from 2009 to 2017, although the underlying reasons remain unknown. Regarding the treatment of refractory KD, the use of third or more IVIG showed a decreasing trend, while the use of corticosteroids and infliximab showed an increasing trend over time. We also confirmed that the incidence rate of CAA was higher in refractory KD patients compared to the overall KD patients. We suggest that continued surveillance is necessary to see whether the proportion of refractory KD continues to increase over time. Early diagnosis and appropriate treatment of refractory KD are essential to prevent CAA, and concomitant infection should be ruled out before making decisions on additional treatments in KD patients who show persistent or recrudescent fever after initial IVIG therapy.







