Introduction
Kawasaki disease (KD) is a self-limited acute febrile illness with an unclear etiology, primarily affecting children under five years of age [1]. Coronary artery aneurysms (CAAs) may occur in up to 25% of patients if KD is left untreated [1]. The overall prevalence of CAAs after KD has been reported to be 1.7%–3.2% [2–4]. Giant CAAs with a diameter greater than 8 mm were identified in 0.09%–0.16% of patients after KD [4,5]. In KD, intimal thickening and calcification may occur at the sites of coronary aneurysms, and luminal myofibroblastic proliferation has been proposed as a potential mechanism for progressive stenosis [6,7]. Changes in flow hemodynamics within CAAs, along with endothelial dysfunction, increase the risk of thrombus formation in patients with KD [8]. Thus, coronary events—including myocardial ischemia due to coronary artery thrombosis, stenosis, or occlusion—can occur in patients with KD during follow-up [9,10]. The risk of coronary events was associated with large CAAs, male sex, and resistance to intravenous immunoglobulin therapy [11]. Symptoms of Myocardial infarction (MI) are often non-specific and difficult to identify, especially in children or infants [1]. Symptoms may include poorly localized pain, unexplained crying, pallor, sweating, and restlessness [1]. In patients with KD and CAAs, the risk of MI peaks within 2 to 3 months after disease onset [1]. Overall, MI occurred in 0.08% of patients with KD in a Taiwanese study [12]. The 10-year survival rate of patients with KD who experienced MI has been reported to be 84.6% [13]. Patients with a reduced left ventricular ejection fraction had significantly worse outcomes, with a 25-year survival rate of 55.3% [13].
Main Subject
The indications for coronary revascularization in KD guidelines were extrapolated from the evidence presented in adult acute coronary syndrome (ACS) guidelines. The American Heart Association (AHA) KD guidelines recommend primary percutaneous coronary intervention (PCI) for patients with ST-elevation myocardial infarction (STEMI) who present to an experienced cardiac catheterization laboratory within 90 minutes [1]. Emergent PCI was recommended for acute MI within 12 hours of onset according to the Japanese Circulation Society (JCS) KD guideline [14]. The discrepancy in time frames between the AHA and JCS KD guidelines stems from the use of different reference points: the AHA KD guidelines base their recommendation on the time from first medical contact, whereas the JCS guidelines use the time from symptom onset. The JCS guidelines also emphasize the importance of timely intervention, recommending primary PCI within 2 hours of hospital arrival. For patients with small body size or those who arrive beyond the recommended time window, systemic thrombolysis or coronary artery bypass grafting (CABG) is recommended [1,14]. Real-world data from Japan suggest that, compared to patients with acute MI primarily due to atherosclerosis, those with KD-associated acute MI were more likely to undergo bypass graft surgery or thrombolysis rather than PCI, with similar in-hospital mortality rates between the two groups [15].
In patients with non-ST-elevation ACS, both the European Society of Cardiology (ESC) and AHA/ACC guidelines recommend determining the invasive strategy based on the patient’s risk profile [16,17]. According to the ESC guidelines for ACS, PCI is considered in stable coronary artery disease when symptoms are not controlled with optimal medical therapy, or when high-risk anatomical features—such as left main disease, three-vessel disease, or proximal left anterior descending disease—are present along with documented ischemia or hemodynamically significant lesions (fractional flow reserve (FFR) ≤ 0.80, instantaneous wave-free ratio (iwFR) ≤ 0.89, or major coronary vessel stenosis > 90%) [17]. The KD guidelines from the JCS also recommend using FFR or iwFR to determine the indication for revascularization when ischemia is not detected by conventional non-invasive tests [14].
Conventionally reported catheter-based treatment options include percutaneous coronary balloon angioplasty, stent implantation, and rotational ablation [18]. The relatively small size of patients presents a challenge for PCI. Additionally, luminal myofibroblastic proliferation and heavy calcification serve as further obstacles to PCI in patients with KD [19]. The following are currently available treatment options for coronary revascularization via PCI.
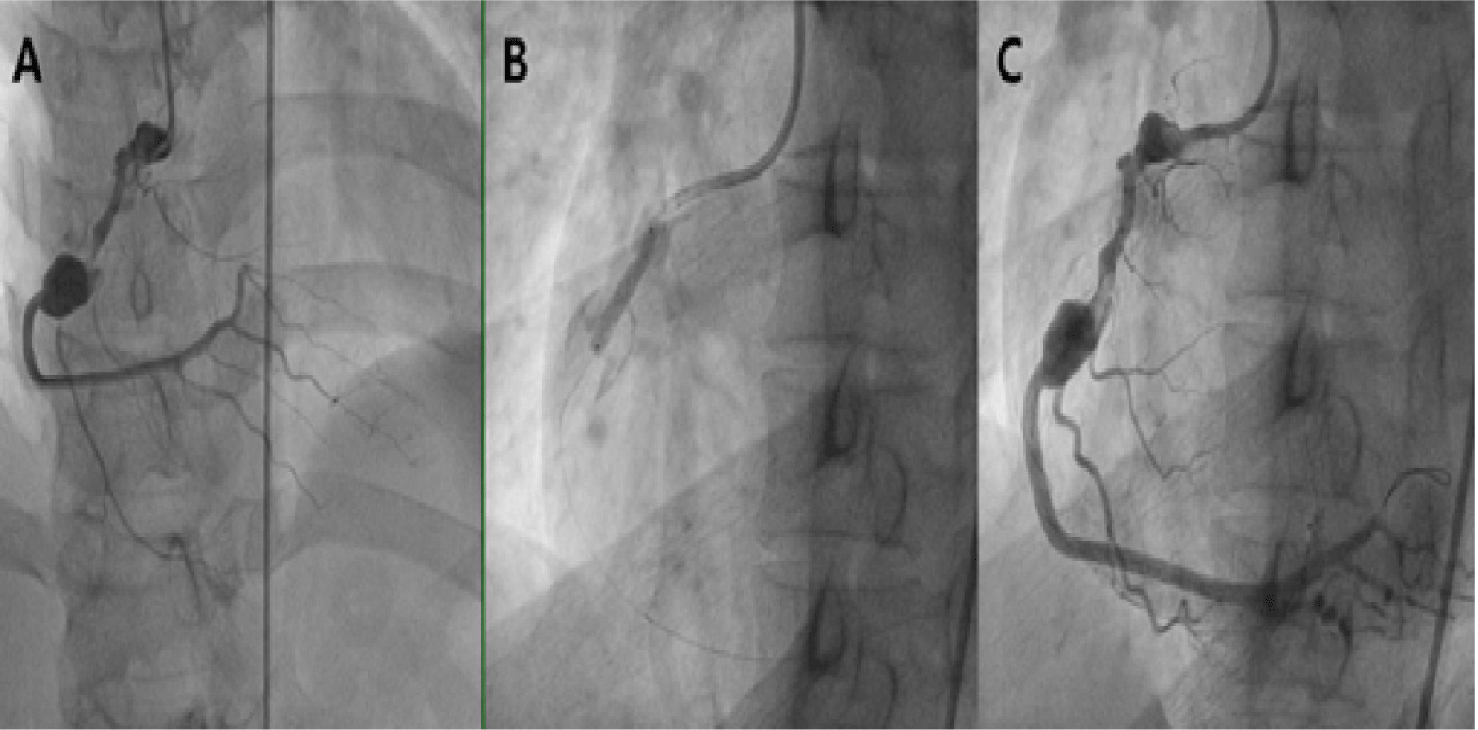
Percutaneous transluminal coronary angioplasty (PTCA) in KD has been reported in the literature since 1988 [18,20–22]. The success rate of PTCA has been reported to be 74%–87% [18,23,24]. While most reports utilized plain old balloon angioplasty, there have been successful cases employing drug-coated balloons or cutting balloons with or without adjunctive rotational atherectomy or excimer laser atherectomy [25–28]. A longer time interval between KD onset and PTCA has been identified as a factor associated with procedural failure [18,22]. Although the pathogenesis of neo-aneurysm formation remains unclear, high-pressure balloon dilatation should be used with caution, as it may contribute to its development [19,21]. Akagi et al. recommended using coronary balloon dilatation at pressures below 10 Atmospheric pressure (ATM), as cases of neo-aneurysm formation were reported following balloon dilatation at pressures of 10 ATM or higher [18,29]. The restenosis rate identified during follow-up after PTCA was 24% [18]. Therefore, restenosis should be carefully monitored after PTCA.
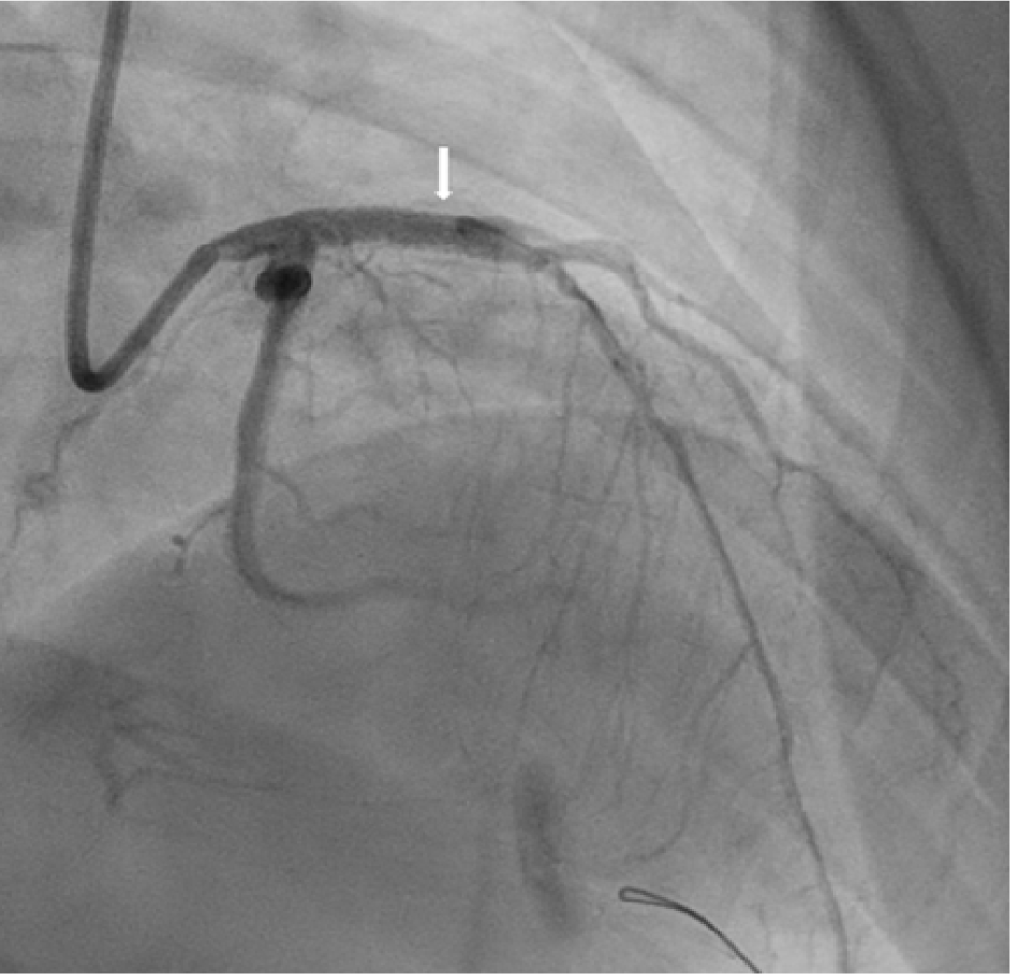
In patients with KD, the immediate procedural success rate of stent implantation has been reported to range from 86% to 100% in a limited number of cases [18,23,24,30–32]. Bare-metal stents, drug-eluting stents (DES), and covered stents have been used [18,23,24,30–32]. The large diameter of CAAs makes safe stent deployment challenging and increases the risk of stent migration in KD-associated coronary lesions [19,33]. Intravascular imaging, using either intravascular ultrasound (Fig. 3) or optical coherence tomography, is crucial for guiding treatment, as thrombus can obscure the true vessel diameter, potentially resulting in undersizing or improper stent placement [19]. Coronary stent implantation was widely regarded as a potential strategy to reduce restenosis and preserve coronary patency after balloon angioplasty [34]. However, a review of the literature revealed that late outcomes following coronary stent implantation were unsatisfactory, with adverse events occurring in 68% of cases, including complete occlusion, restenosis, stent migration, and new aneurysm formation [32]. Accordingly, the JCS guidelines advise against primary coronary stenting in this population [14].
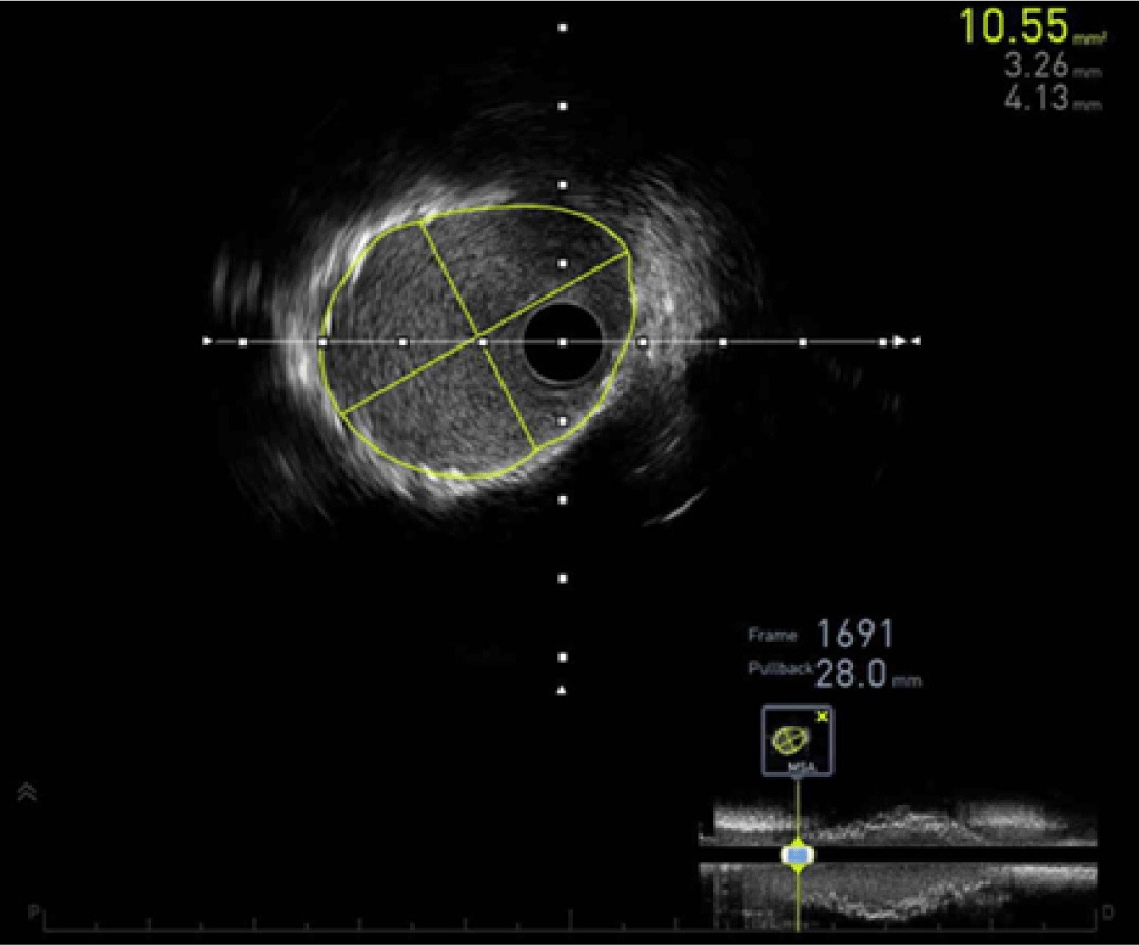
As KD-associated coronary lesions often involve heavy calcification, percutaneous transluminal coronary rotational ablation (PTCRA) has been performed to address these issues [34]. The device features a diamond-coated burr that spins at high speeds (up to 180,000–200,000 revolutions per minute), grinding and breaking down calcified plaque into micro-particles smaller than red blood cells (Fig. 4) [35]. Procedural success rate for the PTCRA has been 93%–100% [18,23,24,26,34,36]. Burr sizes range from 1.25 to 2.5 mm, and the recommended guiding catheter size ranges from 6 to 10 French. Due to the small femoral artery size in small patients, the available burr size is limited, which may affect vessel patency after the procedure [37]. The overall 20-year patency rate was 77% among patients older than 10 years at the time of the procedure [37]. As with other procedures, post-dilation with a balloon at pressures greater than 10 ATM should be avoided due to the risk of neo-aneurysm formation [14]. Although the number of cases was limited, satisfactory results of repeated PTCRA for restenosis have also been reported [37,38]. The JCS guidelines have recommended the use of PTCRA as an appropriate treatment option for calcified stenotic lesions [14]. In addition to PTCRA, a few reports have described the use of excimer laser coronary angioplasty in KD patients as a method for reducing plaque burden in cases of restenosis following stent implantation or complete occlusion [27,39,40].
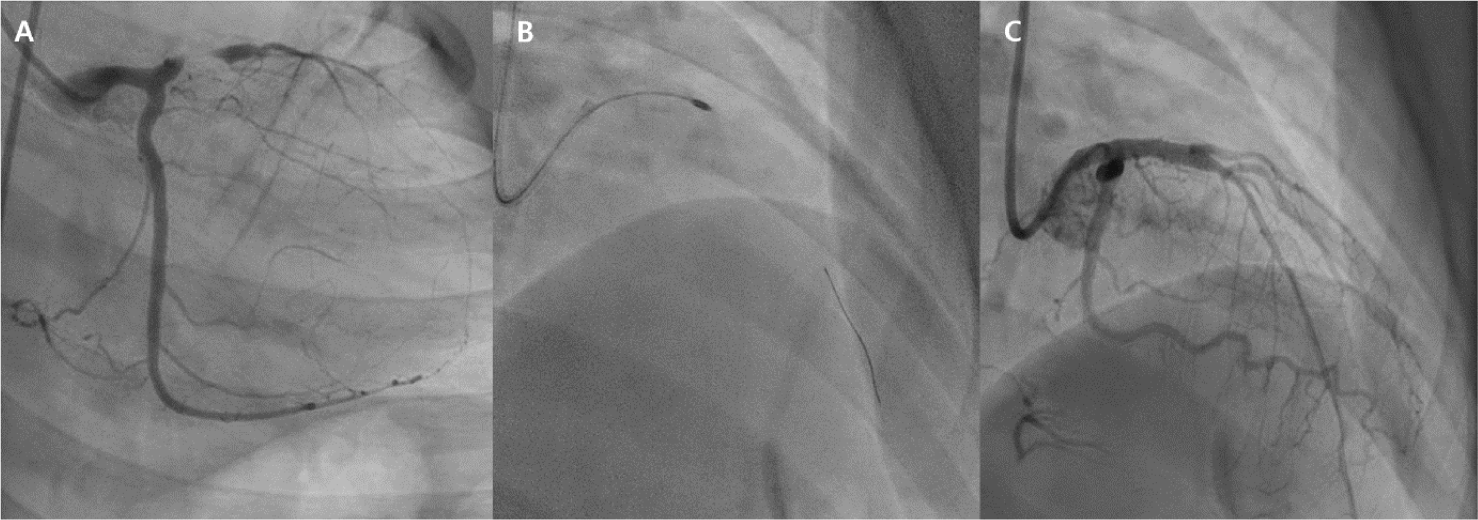
It is often difficult to sufficiently dilate heavily calcified lesions with conventional balloons or stents [41,42]. Also, the use of high-pressure balloons has been associated with neo-aneurysm formation in KD patients [19]. PTCRA may address calcified lesions as mentioned above; however, it is limited to superficial calcium, and deeply seated calcium may be difficult to affect [41,42]. Intravascular lithotripsy can now be used to disrupt calcium in the coronary arteries using sonic pressure waves [41,42]. The energy is delivered through a semi-compliant balloon at low pressure [41]. There was a report of successfully managed KD-related right coronary artery (RCA) stenosis with heavy calcification that was unresponsive to previous PCI with PTCRA and multiple balloon angioplasties, including cutting balloon angioplasty [43]. It is expected to be a useful treatment option for PCI in patients with KD-related coronary arteries with heavy calcification.
Thrombolysis using fibrinolytic therapy is a valuable tool in managing MI related to KD, especially in cases where catheter-based interventions are limited due to the small size of pediatric patients. Theoretically, KD-related ACS is primarily caused by thrombus formation, making thrombolytic therapy a potentially effective treatment option [14]. Serious hemorrhagic events have been reported to be less common in children following thrombolytic therapy [44]. Fibrinolytics can be administered either systemically or via direct infusion into the affected coronary artery through selective coronary catheterization. Although there is a case report of an infant with STEMI in whom intravenous thrombolysis was ineffective but intracoronary infusion proved successful, it remains unclear whether intracoronary or intravenous thrombolysis is superior, and further research is needed to determine the optimal approach [44,45]. In the literature, thrombolytic treatment for ACS associated with KD has been reported to be successful in approximately 47%–67% of patients [23,46–48]. According to the AHA KD guidelines, fibrinolytic therapy is recommended when PCI is not feasible or if the time to PCI exceeds 90 minutes [1]. The JCS KD guidelines recommend systemic thrombolysis when PCI cannot be performed within 12 hours of symptom onset and within 2 hours of hospital arrival [14].
CABG is recommended in ACS patients with multivessel disease or left main disease, considering lesion complexity and patient comorbidities [14,16]. CABG is the treatment of choice for patients with a history of MI, impaired left ventricular function, or coronary lesions not amenable to PCI [14]. The patency of arterial grafts, such as the internal thoracic artery (ITA), radial artery and gastroepiploic artery, is significantly superior to that of saphenous vein grafts [49–51]. Even when CABG was performed in patients younger than 12 years, the 10-year patency rate of the ITA graft remained at 94.4% with appropriate patient selection and the use of PTCA for anastomotic stenosis [52]. Studies comparing outcomes between PCI and CABG in patients with KD have shown no significant differences in survival, although re-intervention rates were higher in the PCI group [53,54]. At 30 years, the survival rate was 93% for patients who underwent single CABG and 91% for those who underwent multiple CABG [50].
From 1998 to 2023, we performed 13 coronary revascularization procedures in 11 patients at our institution. Demographic and angiographic characteristics of patients with aneurysms are summarized in Table 1. The majority were male (n = 8, 72%), and the median age at KD onset was 3.7 years (range, 1.7–6.0 years). Aneurysms involved various coronary segments, most commonly the RCA and the left anterior descending artery (LAD). Giant aneurysms, defined as ≥ 8 mm in diameter, were identified in 7 of the 11 patients (63%). Most patients received dual antiplatelet therapy or a combination of warfarin and aspirin. Procedural data are summarized in Table 2. The median age at the time of the procedure was 10.1 years (range: 3.9–40.6 years). The treated coronary arteries included the RCA in 9 cases, followed by the LAD in 4 cases, the left circumflex artery in 1 case, and the bypass graft anastomosis site in 1 case. Six patients underwent PTCA, including one case involving stenosis at the anastomosis site of a bypass graft. The median follow-up duration after the PCI was 2.9 years (range, 0.7–13.7 years). All PTCA procedures were immediately successful. Although no neo-aneurysm formation was identified on follow-up imaging, interpretation is limited by the short follow-up duration and the lack of imaging data in a substantial proportion of patients (4 of 11). One patient (17%) developed a dissection post-PTCA. Another patient (17%) experienced restenosis of the RCA six months after initial PTCA, requiring repeat PTCA and DES implantation. The restenosis rate after PTCA (1 of 6, 17%) was comparable to that reported in a previous study (24%) [18]. Four patients underwent DES implantation initially—three following PTCA and one PTCRA. One patient (25%) developed total occlusion of the RCA eight months after stent implantation and underwent successful PTCRA. In total, three patients underwent PTCRA, all with immediate procedural success. Two deaths (18%) were recorded during the follow-up period: one unrelated to coronary artery disease and the other in a patient with severe ventricular dysfunction.
Cx: complications; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; DES: drug-eluting stent; PTCRA: percutaneous transluminal coronary rotational ablation; LAD: left anterior descending artery; RCA: right coronary artery; LCx: left circumflex artery; BGA: bypass graft anastomosis; NA: not available; N/A: not applicable; F/U: follow-up.
Conclusion
KD can lead to CAAs, stenosis, and MI, necessitating careful long-term cardiovascular management. In KD patients with coronary artery disease, revascularization strategies—including PCI, thrombolysis, and CABG—are selected based on lesion characteristics, patient age, hemodynamic stability, and timing of presentation. Newer modalities of PCI, such as intravascular lithotripsy, have been introduced to overcome the challenge of heavy calcification in KD-related coronary lesions.







