Introduction
Kawasaki disease (KD) is the leading cause of acquired heart diseases in children in developed countries, particularly in East Asia. South Korea has one of the highest national incidence rates of KD worldwide, highlighting its importance to public health and the need for continuous epidemiological surveillance and clinical research [1].
Over the past two decades, national health databases, such as the National Health Insurance Service (NHIS) and Health Insurance Review and Assessment Service (HIRA), have played a central role in KD research in Korea. These databases provide large-scale population-based data that enable the investigation of incidence, seasonal variation, treatment outcomes, and long-term complications. However, despite their scale and accessibility, these administrative databases have intrinsic limitations, particularly regarding detailed clinical variables, laboratory findings, imaging data, and physician assessment. These limitations restrict the depth of clinical and pathophysiological insights derived from such data.
To overcome these limitations, survey-based datasets curated by the Korea Society of Kawasaki Disease (KSKD) have emerged as valuable complements to administrative data. These surveys provide richer clinical details, often through direct hospital-based reporting or registry frameworks, allowing for studies on coronary artery abnormalities, intravenous immunoglobulin (IVIG) resistance, and other nuanced clinical endpoints. Despite their potential, the scope and impact of KSKD-based studies remain underexplored in relation to the broader landscape of KD research in Korea.
Therefore, we conducted a scoping review of KD-related publications in Korea using national data. By systematically examining study designs, data types, and research topics, we sought to map the evolving trends in KD research and highlight the unique and irreplaceable contributions of survey-based efforts, such as the KSKD survey. The findings of this review are intended to support future methodological decisions and reinforce the scientific rationale for continuing and strengthening disease-specific data collection in Korea.
Methods
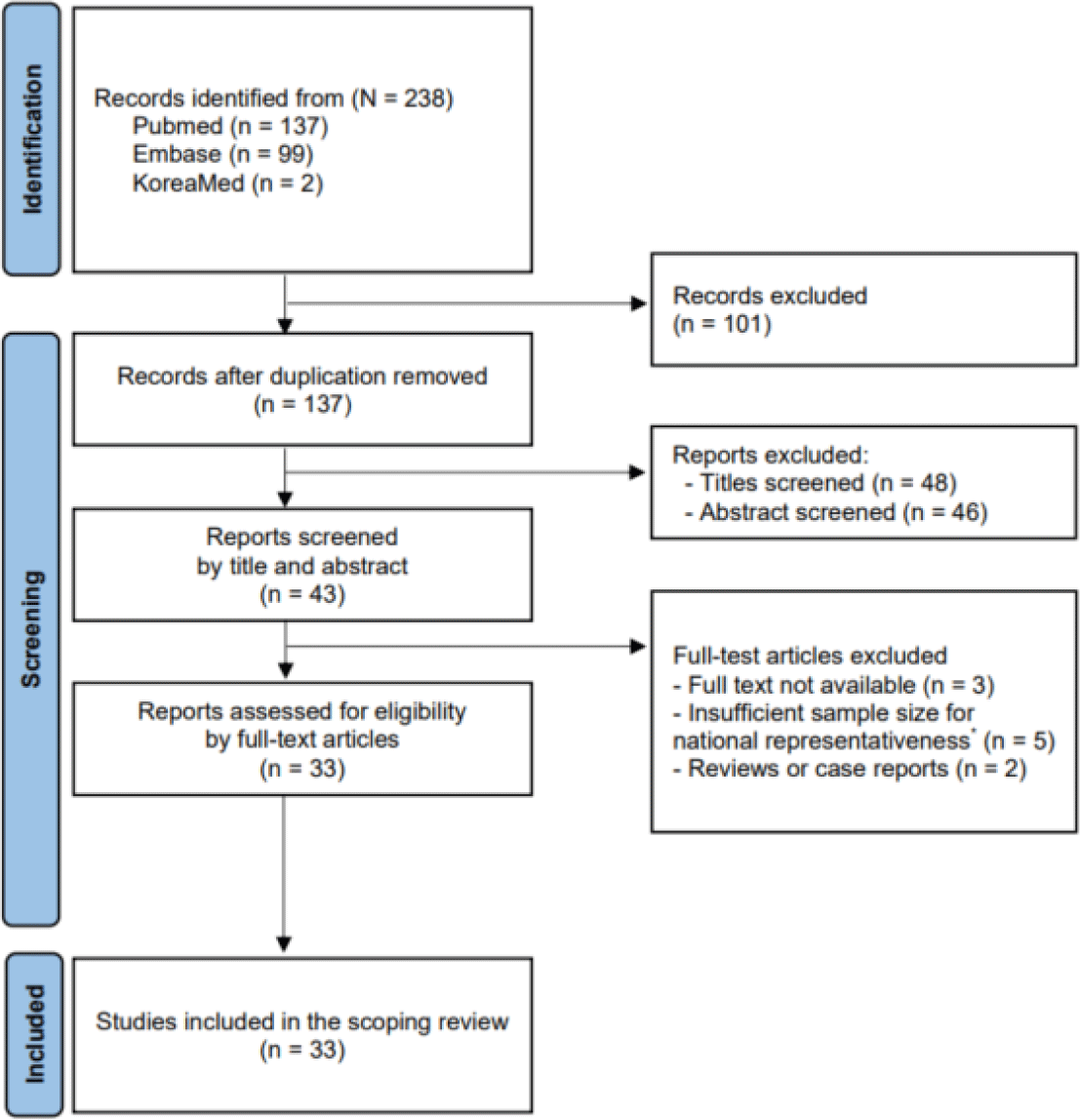
This scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA-ScR) [2]. We systematically searched the PubMed, Embase, and KoreaMed databases for nationwide KD studies conducted in Korea between January 2014 and July 2025. The search period was restricted to 2014–2025 because 2014 marks the beginning of the contemporary phase of nationwide KD research in Korea: nationwide KSKD surveys were standardized during this period, and NHIS/HIRA administrative datasets became more accessible for research following major revisions to data-sharing policies. Earlier publications did not consistently meet nationwide criteria or lacked standardized diagnostic frameworks and therefore were not included. Search terms included combinations of “Kawasaki disease”, “Mucocutaneous lymph node syndrome”, “가와사끼병” or “가와사키병”, “Korea” or “South Korea”, and “national” or “nationwide.” Because this review focused exclusively on studies conducted in Korea, all included articles were published in either English or Korean. Articles were excluded for the following reasons: non-nationwide design, single-center datasets without linkage to a national registry, absence of KD-related outcomes, irrelevant study population, conference abstract only, or non–peer-reviewed material. After the selection and eligibility assessment, 33 studies were included in the scoping review. The literature search and selection processes are illustrated in Fig. 1. The eligibility criteria were established using the Population-Concept-Context (PCC) framework. Given the purpose of scoping reviews, a broad inclusion framework was intentionally applied to map the range of nationwide KD research rather than restricting studies based on methodological uniformity or data quality. This approach aligns with the PCC framework commonly used in scoping review methodology.
-
- Population: Children and adolescents (≤ 19 years) with suspected or confirmed KD in Korea.
-
- Concept: Research focusing on KD using national-level datasets, including administrative databases (e.g., NHIS and HIRA) and nationwide surveys and registries (e.g., KSKD).
-
- Context: Studies conducted in South Korea with a nationwide scope or a nationally representative sample.
-
- Types of sources: Peer-reviewed original studies employing cohort, cross-sectional, case-control, ecological, or hybrid designs. Conference abstracts, commentaries, and single-center studies were excluded, unless they were explicitly linked to a national registry.
Data charting was performed using a standardized extraction form developed and pilot-tested by the review team. For each included study, data were systematically extracted, focusing on bibliographic information (first author, year, and journal), study period, data source (e.g., NHIS, HIRA, Korea Disease Control and Prevention Agency [KDCA], and KSKD survey), study design, study population, case definition and diagnostic codes, main variables, and key findings.
Two reviewers independently extracted the data, and discrepancies were resolved through discussion and consensus. The extracted data were cross-checked for accuracy and completeness before synthesis.
The extracted information was organized into several analytical domains.
-
- Database usage patterns and temporal trends: Frequencies of NHIS, HIRA, KDCA, and KSKD survey use across publication years to identify changes in data utilization over time.
-
- Comparative characteristics of databases: Descriptive synthesis of the strengths and limitations of each data source in terms of accessibility, representativeness, variable richness, and clinical granularity.
-
- Research themes and topic evolution: Categorization of studies by main topic, including epidemiologic trends, environmental or infectious triggers, long-term cardiovascular outcomes, allergic and autoimmune associations, and healthcare utilization, with visualization of shifts in topic emphasis over time.
-
- KSKD nationwide survey summaries: Extraction of key methodological and epidemiological features from historical KSKD surveys to provide context for registry-based trends.
All extracted data were summarized descriptively and presented in tables and figures to highlight temporal and thematic patterns. No quantitative synthesis or formal risk-of-bias assessment was conducted in accordance with the exploratory nature of a scoping review. However, recurring methodological issues, such as reliance on administrative coding, potential case misclassification, and limited linkage with imaging or laboratory data, were qualitatively noted to inform the interpretation of the findings in the Discussion section. Consistent with PRISMA-ScR methodology, no formal risk-of-bias or quality appraisal was performed, as the objective of a scoping review is to map the extent and nature of existing evidence rather than to evaluate study quality or synthesize effect estimates.
Results
A total of 238 records were identified from databases (PubMed 137, Embase 99, and KoreaMed 2). After removing duplicates, 137 records remained, among which 43 titles and abstracts were screened, and 33 full-text articles were assessed for eligibility. Finally, 33 studies were included in the scoping review (Table 1).
| Year | Database | Study design | Topic | Diagnosis | |
|---|---|---|---|---|---|
| Kim et al. [1] | 2014 | KSKD survey | Cohort study | Epidemiology | Clinically diagnosed1) |
| Kim et al. [3] | 2014 | KSKD survey | Ecological time-series study | Epidemiology | Clinically diagnosed |
| Jang et al. [4] | 2015 | KSKD survey | Cohort study | Risk factor analysis | Clinically diagnosed |
| Lee et al. [5] | 2015 | KKDGC | GWAS, case-control study | Gene study | Clinically diagnosed |
| Kim et al. [6] | 2017 | KSKD survey | Cohort study | Epidemiology | Clinically diagnosed |
| Kim et al. [7] | 2018 | KSKD survey | Cohort study | Risk factor analysis | Clinically diagnosed |
| Hur et al. [8] | 2019 | KSKD survey | Cohort study | Treatment outcome | Clinically diagnosed |
| Kim et al. [9] | 2020 | KSKD survey | Cohort study | Epidemiology | Clinically diagnosed |
| Kim et al. [10] | 2021 | KSKD survey | Cohort study | Diagnostic evaluation | Clinically diagnosed |
| An et al. [11] | 2021 | KSKD survey | Cohort study | Risk factor analysis | Clinically diagnosed |
| Kang et al. [12] | 2021 | NHIS | Ecological time-series study | Epidemiology | ICD-10 code-based |
| Choe et al. [13] | 2021 | HIRA | Ecological time-series study | Epidemiology | ICD-10 code-based |
| Kim et al. [14] | 2021 | KKDGC | WES, case-control study | Gene study | Clinically diagnosed |
| Kim et al. [15] | 2021 | KKDGC | GWAS, case-control study | Gene study | Clinically diagnosed |
| Hong et al. [16] | 2021 | Multicenter data | Observation study | Epidemiology | ICD-10 code-based |
| Kwak et al. [17] | 2022 | NHIS | Cohort study, PSM | Risk factor analysis | ICD-10 code-based |
| Na et al. [18] | 2022 | NHIS | Cohort study, PSM | Risk factor analysis | ICD-10 code-based |
| Kim et al. [19] | 2022 | NHIS | Ecological time-series study | Epidemiology | ICD-10 code-based |
| Kang et al. [20] | 2022 | NHIS | Ecological time-series study | Epidemiology | ICD-10 code-based |
| Kwon et al. [21] | 2022 | HIRA | Case-crossover study | Risk factor analysis | ICD-10 code-based |
| Jeong et al. [22] | 2022 | HIRA | Ecological time-series study | Epidemiology | ICD-10 code-based |
| Kim et al. [23] | 2022 | Multicenter data | Observation study | Epidemiology | Clinically diagnosed |
| Cha et al. [24] | 2023 | NHIS | Cohort study | Risk factor analysis | ICD-10 code-based |
| Kim et al. [25] | 2023 | HIRA | Cohort study, PSM | Risk factor analysis | ICD-10 code-based |
| Shin et al. [26] | 2023 | HIRA | Cohort study, PSM | Risk factor analysis | ICD-10 code-based |
| Kim et al. [27] | 2024 | NHIS | Cohort study, longitudinal | Risk factor analysis | ICD-10 code-based |
| Oh et al. [28] | 2024 | HIRA | Cohort study, PSM | Epidemiology | ICD-10 code-based |
| Kim et al. [29] | 2024 | HIRA | Cohort study, PSM | Comorbidity analysis | ICD-10 code-based |
| Yu et al. [30] | 2024 | Multicenter data | Observation study | Diagnostic evaluation | Clinically diagnosed |
| Cheon et al. [31] | 2025 | KSKD survey | Cohort study | Risk factor analysis | Clinically diagnosed |
| Kwak et al. [32] | 2025 | NHIS | Cohort study | Comorbidity analysis | ICD-10 code-based |
| Lee et al. [33] | 2025 | NHIS | Cohort study, PSM | Comorbidity analysis | ICD-10 code-based |
| Lee et al. [34] | 2025 | HIRA | Cohort study | Treatment outcome | ICD-10 code-based |
KSKD: Korea Society of Kawasaki Disease; KKDGC: Korean Kawasaki Disease Genetics Consortium; GWAS: genome-wide association study; NHIS: National Health Insurance Service; HIRA: Health Insurance Review of Assessment Service; ICD: international classification of diseases; WES, whole exome sequencing; PSM: propensity score matching.
Over the past decade, the use of national databases for KD research in Korea has undergone significant changes. Early investigations (2014–2018) were primarily derived from the KSKD survey, which provided clinician-reported nationwide epidemiological data. These survey-based studies laid the foundation for understanding the demographic and seasonal patterns of KD but were limited by their sample size and reporting frequency, as shown in Fig. 2.
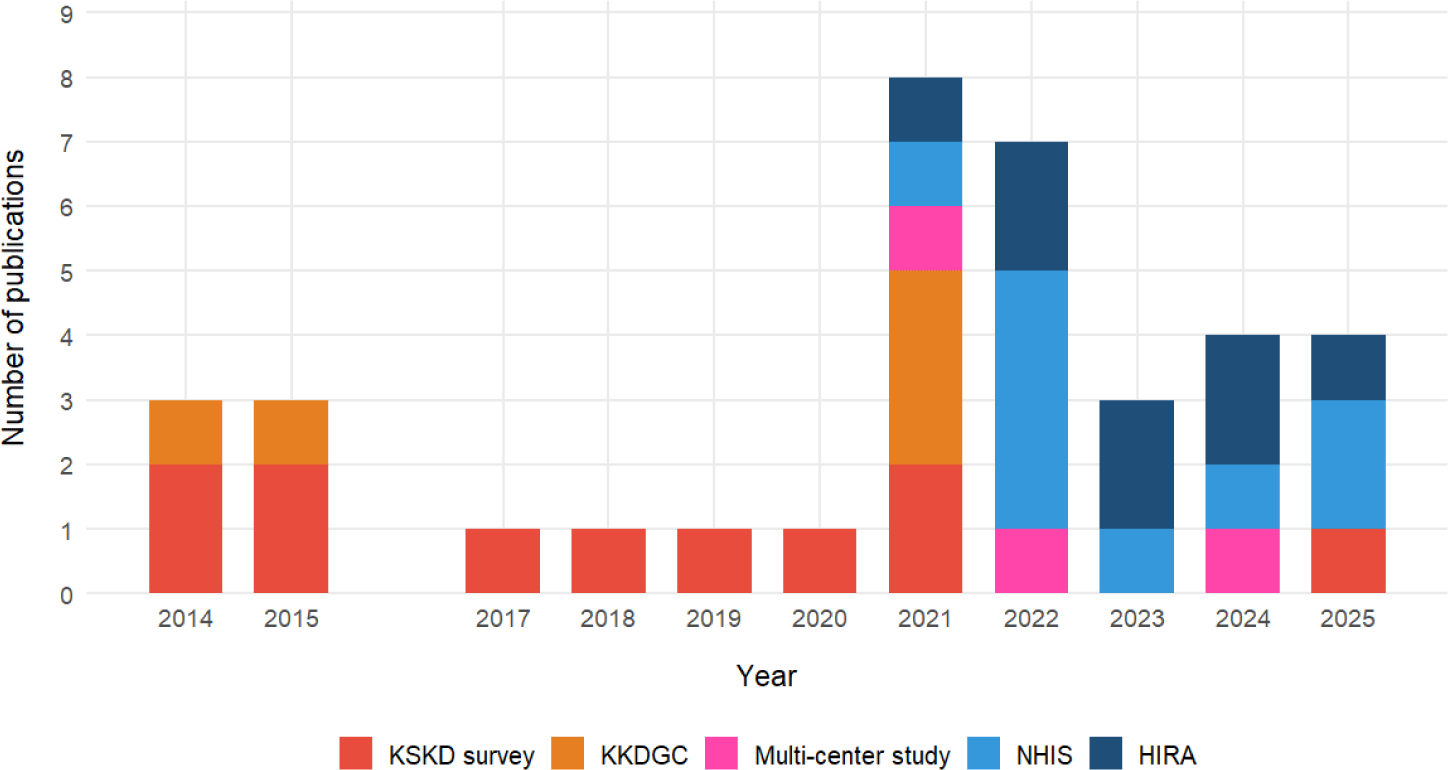
From 2019 onward, there was a noticeable transition toward the utilization of large-scale administrative datasets, particularly those from the NHIS and HIRA. These databases enable longitudinal tracking of patients, detailed analyses of treatment patterns, and outcome-based studies using methods such as propensity score matching. By 2021–2025, the NHIS and HIRA had become the dominant data sources for KD research, marking a clear shift from descriptive surveys to comprehensive, data-driven epidemiological and clinical studies. Meanwhile, the KSKD survey retained its complementary role by providing detailed clinical information, such as IVIG response and coronary artery outcomes, which the administrative datasets could not capture.
The distribution of study designs varied according to the data source (Fig. 3). Clear patterns in the study design were observed across data sources. Survey-based studies, such as those based on the KSKD, primarily employed retrospective cohort or cross-sectional designs, focusing on clinical characteristics, treatment response, and coronary complications. These studies were typically hospital-driven and relied on physician-confirmed diagnoses, allowing detailed evaluation of clinical endpoints, but with limited generalizability due to smaller and non-continuous samples.
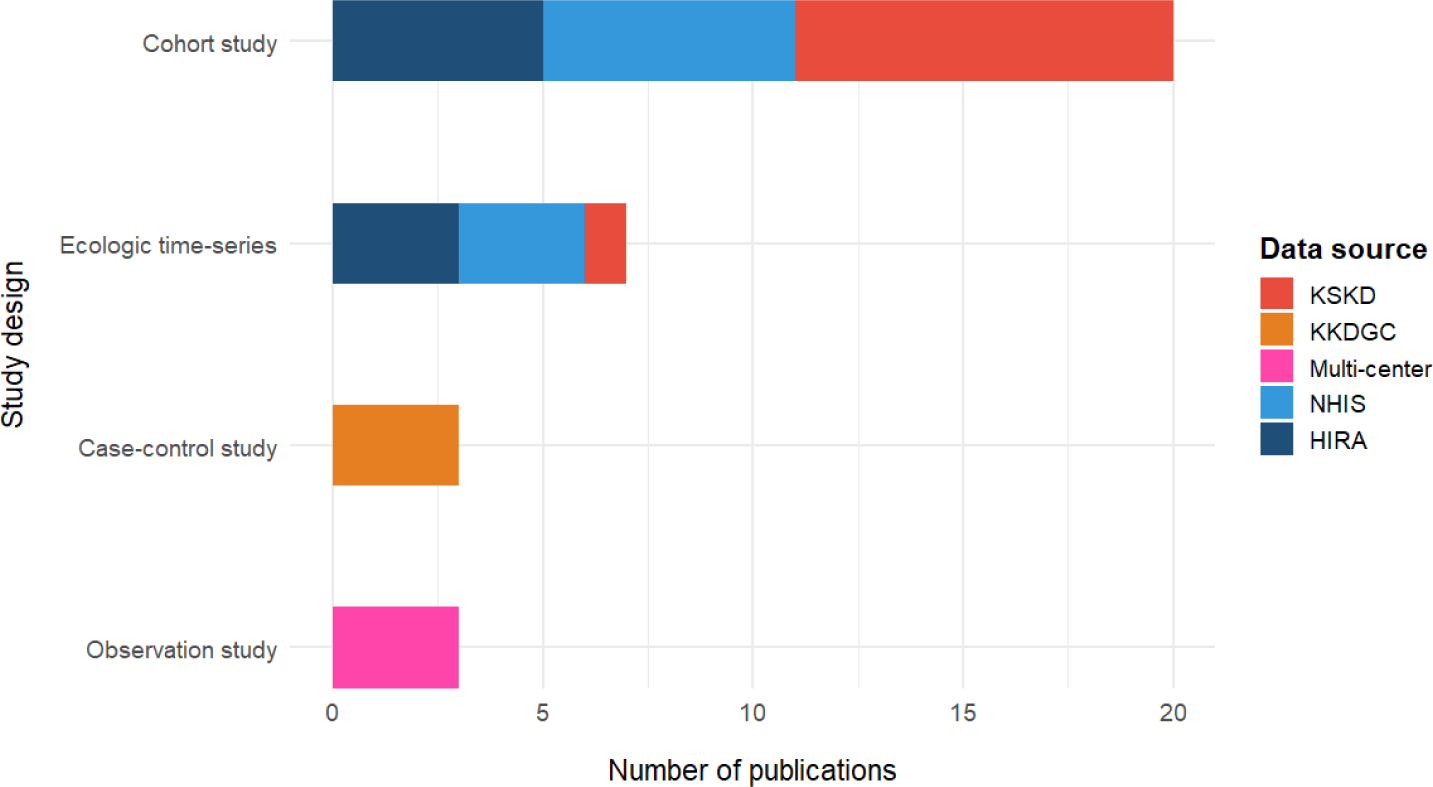
In contrast, the NHIS- and HIRA-based studies predominantly utilized large-scale cohort or case-control designs, often incorporating propensity score matching or longitudinal follow-up, to examine treatment outcomes and long-term comorbidities. Ecological time-series and case-crossover designs were also frequently applied in environmental epidemiology studies, particularly in those assessing the short-term effects of air pollution on KD incidence. The diversity of study designs across databases reflects evolving research objectives, from clinician-driven descriptive analyses to population-based causal inference and policy-relevant modeling.
The thematic landscape of KD research in Korea has broadened considerably over time. In the early period (2014–2018), most studies focused on epidemiology, including incidence, seasonal variation, and demographic characteristics, based on the KSKD survey data. Research began to diversify between 2019 and 2021, with an increasing number of studies on risk factors, environmental triggers, and genetic susceptibility, supported by the analytical power of the NHIS, HIRA, and Korean Kawasaki Disease Genetic Consortium (KKDGC) datasets.
Recently (2022–2025), studies have increasingly addressed treatment outcomes, comorbidity analysis, and diagnostic evaluation, reflecting a transition toward more mechanistic and clinically relevant questions. Notably, environmental and immunological studies have linked KD to air pollution and allergic diseases. This temporal evolution of research topics mirrors the expanding capacity of national databases and the integration of multidomain approaches, from epidemiological surveillance to clinical and translational research, within KD science in Korea.
Discussion
This scoping review summarized nationwide KD research conducted in Korea over the past decade and described the evolution of study designs and data utilization. Early investigations mainly relied on clinician-reported surveys by the KSKD, which provided the first nationwide data on the incidence, clinical features, and coronary outcomes. With the subsequent availability of large-scale administrative databases, particularly the NHIS and HIRA, KD research in Korea has shifted toward population-based cohort analyses. These studies have enabled the longitudinal tracking of patients, evaluation of treatment outcomes, and assessment of long-term cardiovascular risks. This transition from survey-based descriptive studies to data-driven hypothesis-oriented analyses reflects the methodological maturation of KD research in Korea.
Distinct differences in study design were observed across various data sources. The KSKD survey, while often described as nationwide in scope, is a clinician-reported dataset with voluntary participation rather than a complete national census; thus, it should be interpreted as a broad multi-center nationwide survey rather than a fully population-representative registry. In addition, participation rates in the KSKD survey vary across years, and the completeness of reporting differs by institution, which may limit the consistency and generalizability of survey-based estimates. As a retrospective clinician-reported dataset, the KSKD survey is also subject to variations in diagnostic practices and data entry across hospitals.
In contrast, the NHIS and HIRA data allowed for comprehensive epidemiological studies using robust analytic frameworks, such as propensity score matching, longitudinal follow-up, and ecological or case-crossover analyses. The complementary roles of these data sources highlight how a combination of administrative- and registry-based research can provide both breadth and depth for understanding the epidemiology and outcomes of KD. Furthermore, the lack of validation studies for KD coding algorithms in NHIS and HIRA remains a critical methodological gap. Without linkage to clinical registries, it is not possible to determine the sensitivity, specificity, or positive predictive value of code-based KD case definitions. Misclassification may distort incidence estimates and bias associations with comorbidities, environmental exposures, or long-term outcomes. The challenge is even greater for coronary artery aneurysms, which require echocardiography-based Z-scores that are not available in claims data.
The clinical depth of the KSKD registry illustrates why registry-based data are essential for validating and refining these definitions. For example, definitions of IVIG resistance differ across hospitals, and the calculation of fever days varies depending on whether subjective fever, thermometer-confirmed fever, or emergency department temperature is included—details that cannot be captured in administrative datasets. Coronary artery abnormalities also depend on standardized Z-score calculations based on body surface area and measurement protocols, all of which require imaging data absent from NHIS and HIRA. In contrast, the KSKD registry contains physician-adjudicated diagnoses, fever-day documentation, and echocardiographic measurements, enabling accurate characterization of IVIG resistance, acute disease severity, and coronary outcomes. Together, these examples underscore the indispensable role of clinically rich registries in complementing administrative data and mitigating misclassification biases in KD research.
This review has several strengths. To the best of our knowledge, it is the first study to comprehensively map national-level KD research in Korea across all major databases, providing an integrated overview of the evolution of the research landscape. By categorizing studies according to data source, design, and topic, this review offers a systematic framework for understanding methodological diversity and database-specific capabilities. Furthermore, it underscores the continuing relevance of the KSKD registry as a clinically validated dataset that complements the scale of administrative data.
However, this study also had several limitations. As a scoping review, it did not include quantitative synthesis or formal quality assessment. The included studies were heterogeneous in design and population coverage, limiting direct comparisons. In addition, the search was confined to indexed literature, which may have excluded unpublished data or conference proceedings. The reliance on diagnostic coding in administrative databases may also have introduced case misclassification. A fully systematic comparison of variable structure, coverage rate, and coding accuracy across data sources was not feasible because such metadata are not publicly available for NHIS and HIRA and are not consistently reported for the KSKD survey. Therefore, our comparison focused on characteristics that could reliably be extracted from published studies—such as study design, clinical depth, and the suitability of each data source for epidemiologic versus clinically oriented research questions.
The findings of this review have several implications for future KD research. Korea’s national health data infrastructure offers a unique opportunity to conduct large-scale epidemiological studies and detailed clinical investigations. In addition to the expansion of administrative data resources, several contextual factors may also have contributed to the observed shift in research topics over time. Updates to KD diagnostic guidelines, changes in the research environment during the COVID-19 pandemic, evolving national funding priorities, and broader health-policy reforms may all have influenced the types of scientific questions pursued by investigators. Therefore, the temporal evolution of KD research should be interpreted as the result of multiple interacting forces rather than data availability alone.
Building on these observations, several concrete priorities emerge for future nationwide KD research. Establishing a dual-track approach that integrates administrative databases with registry-based clinical data will enhance both statistical power and clinical validity. Validation studies linking NHIS/HIRA claims with clinically adjudicated registries are needed to improve the accuracy of KD and coronary aneurysm case definitions. Developing a standardized national clinical registry with consistent participation and harmonized coronary Z-score reporting would enable more reliable assessment of treatment response and cardiovascular outcomes. Incorporating imaging, laboratory, and biomarker data into nationwide datasets would markedly deepen the clinical resolution of future studies. Long-term cardiovascular follow-up through multi-source linkage is essential to characterize late complications. Finally, the establishment of a real-time or near–real-time KD surveillance platform could facilitate the early detection of epidemiologic changes and support timely public-health responses. These future directions collectively highlight the importance of sustained investment in integrated, clinically rich nationwide KD data systems.
Conclusion
Korea has established one of the most comprehensive infrastructures for nationwide KD research by integrating large-scale administrative data with clinically detailed registries. Although administrative databases have greatly advanced KD research, they cannot substitute for clinically detailed registries. A dual-track approach that leverages the scale of administrative data and the depth of clinical registries is essential for improving patient outcomes and guiding evidence-based health policies.
