Introduction
Kawasaki disease (KD) is a systemic vasculitis. It rarely accompanies severe complications such as giant coronary artery aneurysm (CAA) that can cause thrombosis or stenosis resulting in myocardial infarction. Here, we report a case of an infant who received rescue coronary artery bypass grafting (CABG) at 10 months of age to relieve myocardial ischemia due to compression of the LAD artery by giant left main CAA which was developed after diagnosis of KD at 8 months of age.
Case Report
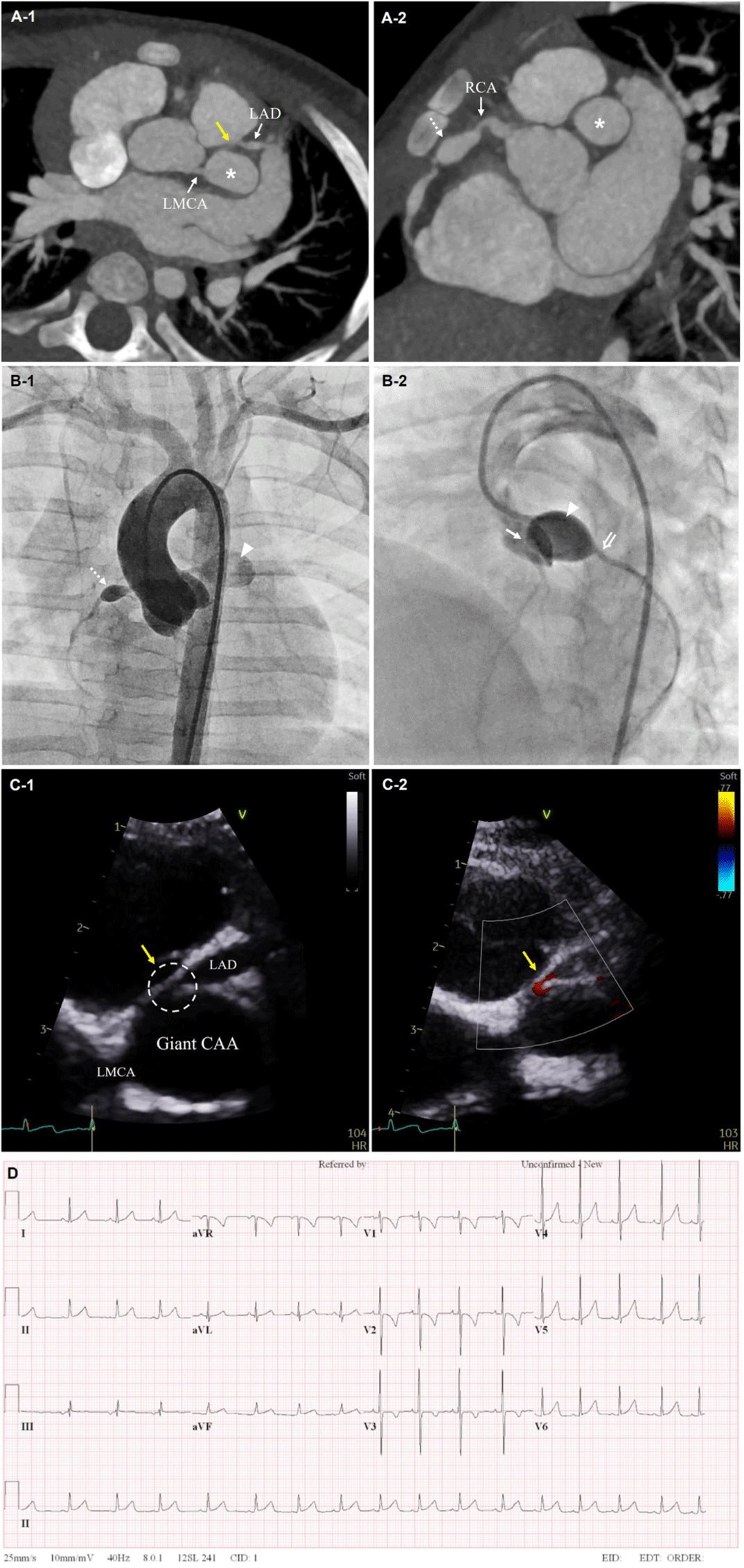
An 8-month-old boy received two cycles of intravenous immune globulin and three days of methylprednisolone pulse therapy under the diagnosis of KD. During this treatment, a giant CAA in distal left main coronary artery (LMCA; 10.5 mm, z = 25.4), a tortuous fusiform LAD artery (3.8 mm, z = 7.7), and a fusiform mid right coronary artery (RCA; 5.7 mm, z = 13.1) with luminal irregularity were observed. Therefore, dual antiplatelet (aspirin and clopidogrel) and anticoagulant (warfarin) were prescribed and serial echocardiography was performed in a short-term period. Giant CAA was continuously observed and a beta-blocker was added. Ten days after the aneurysm was first identified, a cardiac computed tomography (CT) was performed for suspected intramural thrombosis in the LAD artery from echocardiography. The cardiac CT scan (Fig. 1A) showed no thrombus in the coronary artery, but a giant CAA of distal LMCA, proximal ostial stenosis of LAD artery, and RCA fusiform aneurysm were identified. Coronary angiography was subsequently performed for further evaluation. The results showed a very dilated distal left main CAA (9.7 × 14 mm) resulting in kinking of the LAD artery and left circumflex arteries (LCx), and severe stenosis of the proximal LAD artery with sluggish blood flow to the LAD artery (Fig. 1B). The patient was followed up with echocardiography every 2–3 days to observe changes in the proximal LAD artery stenosis while maintaining close observation of symptoms of myocardial ischemia with electrocardiography and cardiac enzymes. At 4 weeks follow-up, proximal LAD artery stenosis was greater than 80% and decreased blood flow towards the LAD artery was observed in echocardiography (Fig. 1C). There were no significant changes in cardiac enzymes suggesting myocardial ischemia, but ST changes were observed on the electrocardiography in leads II, aVF (Fig. 1D).
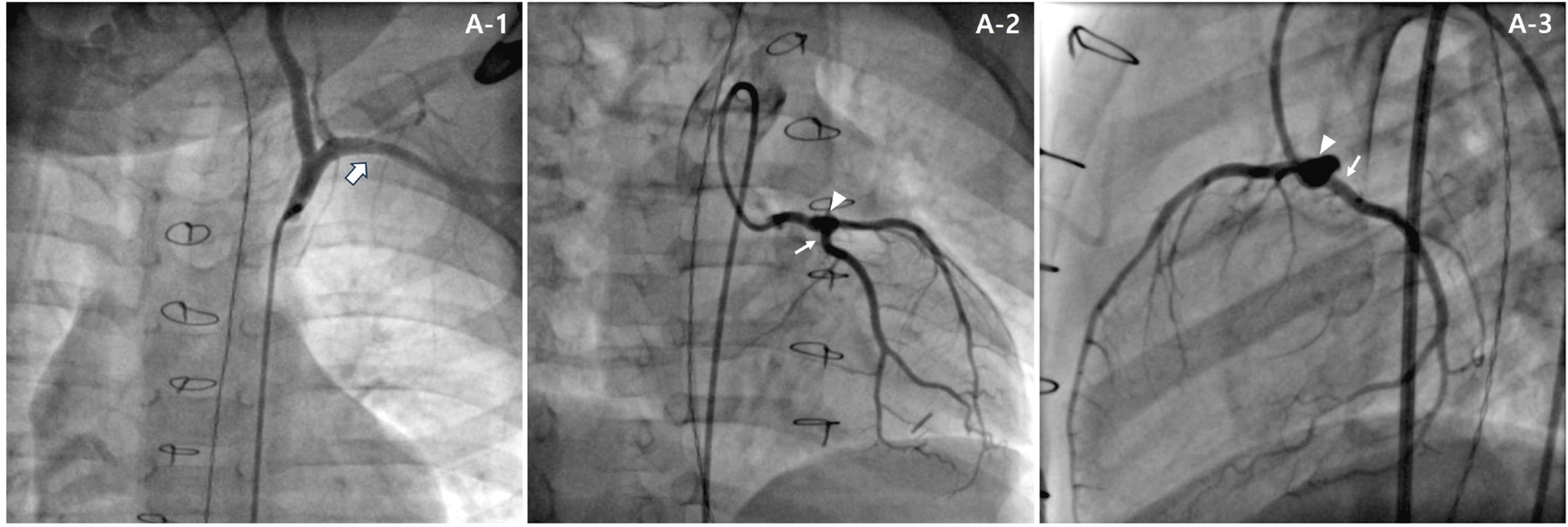
A rescue conventional CABG using the left internal thoracic artery was planned to prevent the potential risk of myocardial infarction. The left internal thoracic artery was connected to a non-stenotic area distal to the stenosis in the LAD artery and there were no complications. Post-operatively, the patient’s hemodynamic status remained stable, and he was transferred to the general ward after 24 hours of observation in the intensive care unit (ICU). A cardiac CT scan performed on postoperative day 6 showed good patency of the coronary artery bypass graft. On postoperative day 13, the patient was discharged with aspirin, clopidogrel, warfarin, and atenolol without any symptom. Elective cardiac CT performed at 15 months after CABG showed the absence of graft flow, although the patient did not have any ischemic symptom. We performed coronary angiography and found that distal left main CAA had regressed to 5.8 mm and the proximal LAD artery also showed patent forward flow without stenosis (Fig. 2A). There was also no forward coronary artery bypass graft flow. Therefore, we discontinued warfarin and he is being followed up in an outpatient clinic without any symptom on aspirin and clopidogrel.
Discussion
CAA is a representative complication associated with KD and shows an incidence of approximately 5% in patients treated with intravenous immune globulin and 20%–30% in untreated patients. Aneurysm exhibits a wide range of remodeling processes such as regression, persistent aneurysm, and localized stenosis [1]. The regression of aneurysm depends on its size. Giant CAA with internal diameter of 8 mm or more rarely regresses, and even if it does, the regression process may cause intimal thickening, resulting in progressive stenosis or occlusion [2,3]. Giant CAA also shows a decrease in blood flow velocity, which induces endothelial dysfunction associated with thrombosis and raises the possibility of thrombotic coronary occlusion [3,4]. Active treatment of giant CAA is crucial since coronary artery stenosis or occlusion may progress to ischemic heart disease [5].
It has been reported so far that stenoses of coronary arteries associated with giant aneurysms in KD are mostly caused by intimal thickening of the arteries or thrombus formations by an inflammatory processes [2,4]. Although rare, giant aneurysms compressing adjacent cardiac structures have also been reported, such as a case of a giant right CAA causing severe compression of the right heart and leading to cardiopulmonary symptoms [6]. Here, we report a case of an infant Kawasaki disease patient with a giant CAA at LMCA that caused compression and significant stenosis at adjacent coronary artery.
In acute phase, the patient showed a severely dilated giant aneurysm in the distal LMCA without thrombosis or stenosis; however, the aneurysm compressed the proximal LAD artery and LCx leading to 80% stenosis at proximal LAD artery. Fortunately, forward flow in the proximal LAD artery was spontaneously restored as the giant left main CAA regressed and subsequently relieved the proximal LAD artery stenosis. This suggests that giant aneurysm-associated LAD artery stenosis was caused by mechanical compression of the LAD artery by a severely dilated left main CAA during acute phase rather than by inflammatory change. CABG was a definite rescue treatment for this infant patient. In children, CABG using the internal thoracic artery with its capacity for long-term patency and adaptation for rapid somatic growth is the standard treatment for severe proximal LAD artery stenosis associated with giant aneurysm [1,3,7–9]. It was reported that the mean age of patients at operation for CABG was about 10 years and the youngest was 19 months old [9]. CABG for young children is usually conducted when they are older than 1 year due to technical difficulties related to the small size of the coronary arteries [9]. However, we report a successful performance of CABG in a 10-month-old Kawasaki disease patient. It implies that, if needed, CABG may be used for treatment of giant CAA and adjacent coronary artery stenosis to prevent the risk of impending myocardial infarction even in patients younger than 1 year old.
After CABG using internal thoracic artery, the graft shows long-term patency when good blood flow is observed at early post-operative phase. Graft occlusion may occur mainly due to stenosis of the anastomosis site or competition of the forward flow in the graft and the anastomosed coronary artery [7]. In our case, the internal thoracic artery graft showed good patency initially at early postoperative phase but graft flow disappeared during follow-up. The disappearance of graft forward flow appears to be caused by the competition between graft flow and improved native flow in the LAD artery, with relief of the LAD artery ostial stenosis after the regression of the giant CAA in the distal LMCA. Thus, it can be assumed that the loss of coronary artery bypass graft forward flow was induced by functional occlusion rather than physical occlusion. This is supported by a previous report on a patient having undergone CABG with an internal thoracic artery graft, where flow through the graft was absent while native coronary flow was maintained, but reappeared 10 years after surgery when native coronary flow was lost [6].
In summary, the present case suggests that a severely dilated distal LMCA during the acute phase of Kawasaki disease can compress the proximal LAD artery or LCx, causing ischemic symptoms, and that CABG could be a rescue treatment in such acute settings even in small infants.
